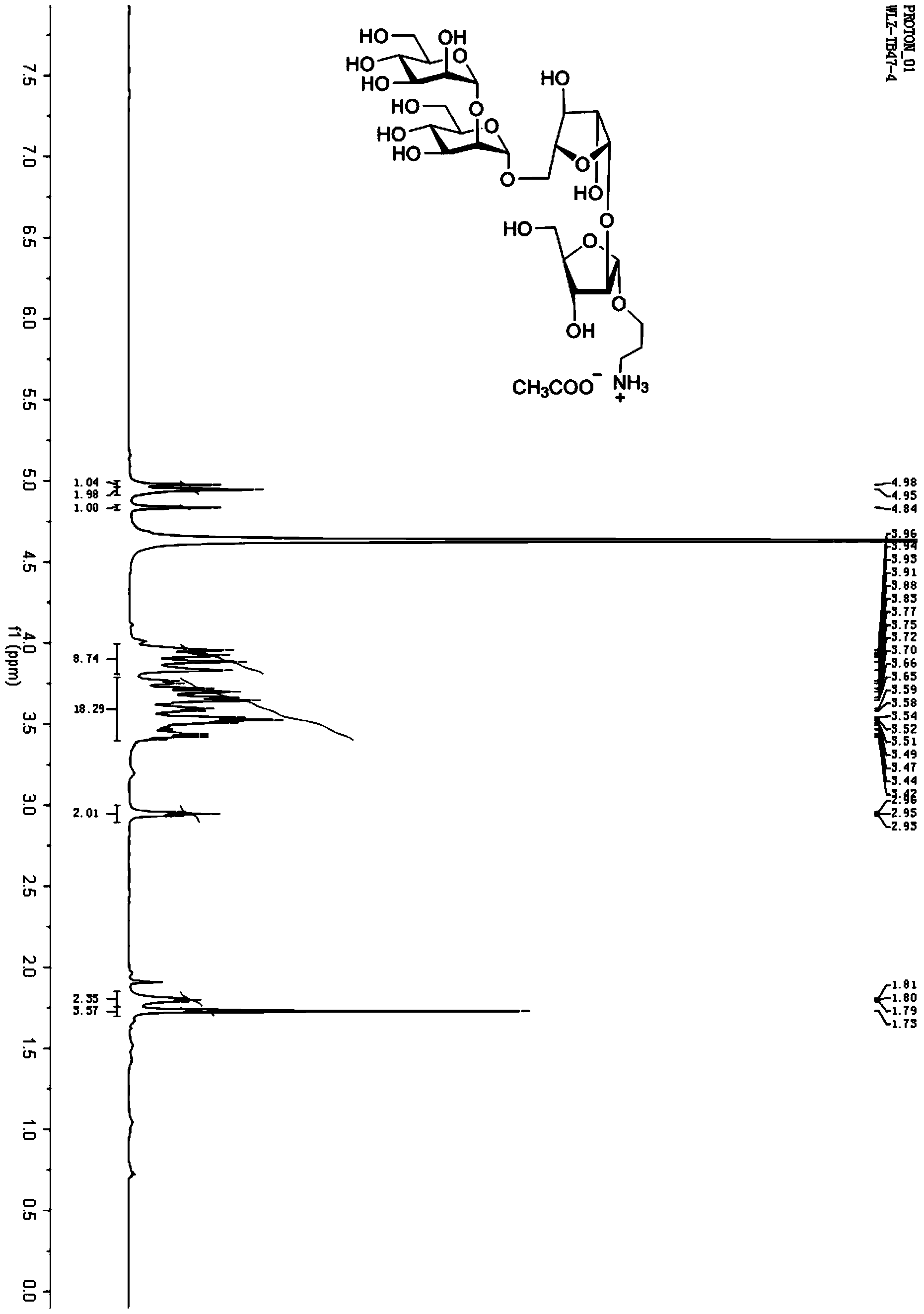
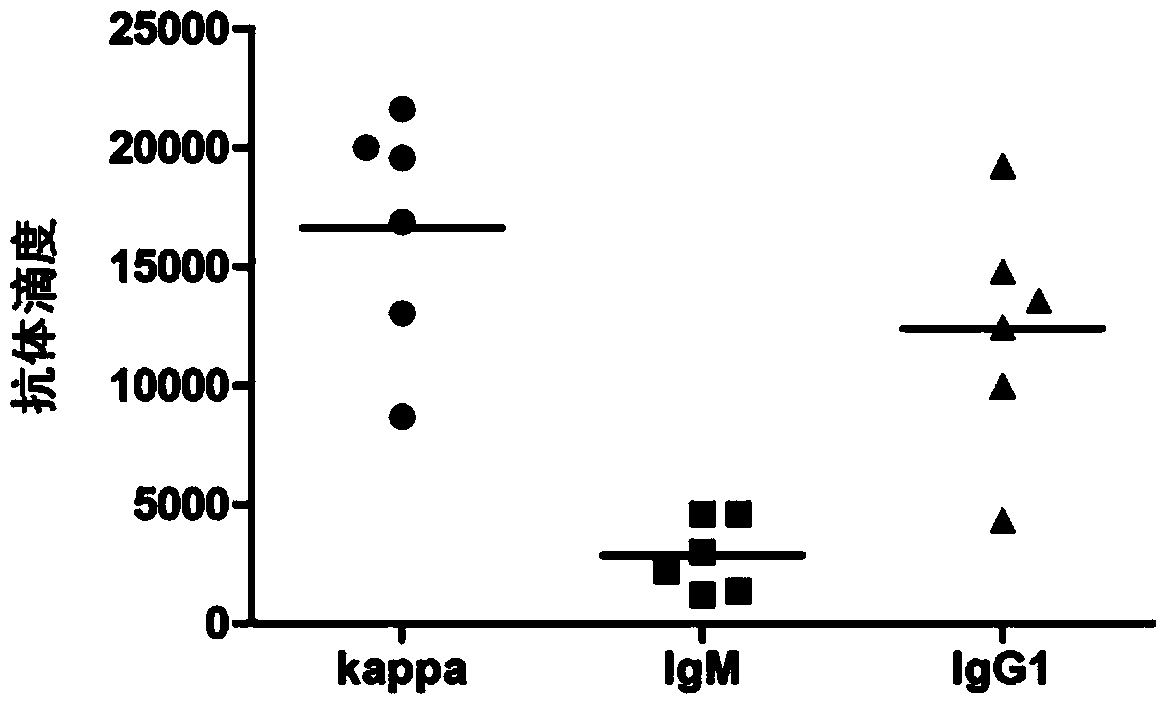
Mycobacterium tuberculosis LAM oligosaccharide conjugate as well as preparation method and application thereof
A technology of Mycobacterium tuberculosis and conjugates, applied in chemical instruments and methods, carrier-bound antigen/hapten components, antibacterial drugs, etc., to achieve good immune effect, avoid bacterial drug resistance, and solve the effect of low immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: general synthetic method
[0035] A: Glycosylation reaction
[0036] Take the glycosyl donor (1.2 equivalents), the glycosyl acceptor (1 equivalent) and the molecular sieve (equal to the weight of the donor) and dissolve them in dry anhydrous dichloromethane, and cool the reaction solution to - After stirring at 30°C for 30 minutes, N-iodosuccinimide (NIS, 1.2 equivalents) and a catalytic amount of silver trifluoromethanesulfonate (AgOTf) were added. The reaction solution was slowly raised to room temperature, neutralized with triethylamine after TLC detected that all the raw materials had reacted, filtered to remove insoluble solids, the filtrate was spin-dried, and the crude product was separated through a silica gel column to obtain the target product (the eluent used was petroleum ether (PE ) / ethyl acetate (EA), unless otherwise stated).
[0037] B: deacetylation reaction
[0038] Dissolve the raw materials in methanol, add 1mol / L sodium methoxide m...
Embodiment 2
[0046] Example 2: 3-aminopropyl α-D-mannosyl-(1→2)-α-D-mannosyl-(1→5)-β-D-arabinosyl-(1→2) -Synthesis of α-D-arabinose-KLH conjugate (A11-KLH)
[0047] (1) Synthesis of 5-O-tert-butyldimethylsilyl-D-arabinose (A1)
[0048]
[0049] Dissolve D-arabinose (5.00g, 33mmol) in 100mL of pyridine, add tert-butyldimethylsilyl chloride (6.00g, 40mmol) and a catalytic amount of 4-dimethylaminopyridine at 0°C, and dissolve the reaction solution Stir at room temperature for 6 hours. The reaction was tracked by TLC (EA). After all the raw materials were reacted, the solvent was evaporated to dryness to obtain a yellow viscous liquid. The crude product was separated by a silica gel column to obtain a colorless oily liquid (6.34 g, yield 72%).
[0050] (2) Synthesis of 1,2,3-tri-O-acetyl-5-O-tert-butyldimethylsilyl-α,β-D-arabinose (A2)
[0051]
[0052] Take A1 (5.00g, 19mmol) and dissolve it in a mixed solvent of 5mL acetic anhydride and 20mL pyridine, cool the mixed solution to 0°C...
Embodiment 3
[0083] Example 3: 3-aminopropyl α-D-mannosyl-(1→2)-α-D-mannosyl-(1→5)-β-D-arabinosyl-(1→2) -Synthesis of α-D-arabinose-BSA conjugate (A11-BSA)
[0084]
[0085] Take A11 (1 mg) and BSA (3 mg) to synthesize A11-BSA conjugate (2.7 mg) according to the general synthesis method E in Example 1. Mass Spectrum: MALDI-TOF MS (m / z): 74354
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com