Targeted ligand-PEG (polyethylene glycol)-cholesterol/tocopherol derivative, and preparation method and application of derivative
A technology of cholesterol derivatives and targeting ligands, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and drug combinations, etc., which can solve the problem of unsatisfactory tissue selectivity, affecting drug delivery efficiency, and weak microenvironmental responsiveness And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of anti-EphmAb-polyethylene glycol-cholesterol and preparation of DOX / siRNA cationic liposomes and pharmacy / biological investigation
[0058] 1) Ep10mAb-PEG 2000 Synthesis of -SH
[0059] Bifunctional PEG 2000 Dissolve in water, add a certain amount of EDC and S-NHS, react at room temperature, dialyze to remove excess product, add a certain amount of Ep10mAb to the reaction solution, and react in the dark to prepare Ep10mAb-PEG 2000 -SH dialyzed to remove excess reactants, freeze-dried. After the product was purified, the structure of the product was confirmed by infrared spectroscopy and nuclear magnetic resonance spectroscopy. Then, electrophoresis and ELISA methods were used to investigate the effect of the antibody linked with PEG on its activity and the effect of the linked antibody on tumor cell proliferation.
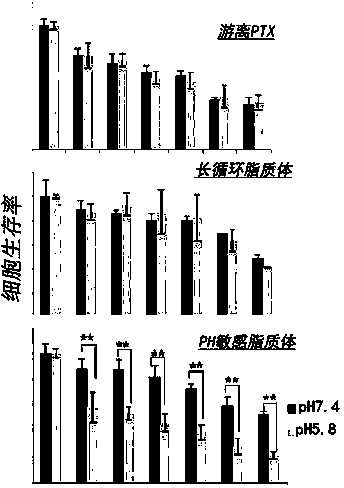
[0060] 2) Synthesis of pH-sensitive cholesterol derivatives
[0061] Dissolve an appropriate amount of cholesterol chloroformate...
Embodiment 2
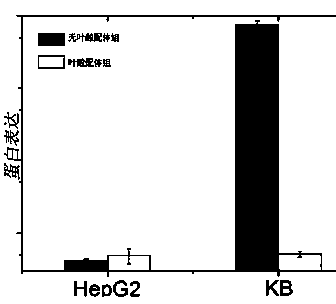
[0064] Example 2: Synthesis of folic acid-PEG-cholesterol derivatives, construction of self-assembled carriers, formulation and biological research.
[0065] 500 mg of folic acid and NHS were dissolved in 10 ml of DMSO, excess DCC was added to the solution and stirred overnight at room temperature, and the insoluble dicyclohexylurea was removed by suction filtration. Folate-NHS and bisamino-PEG were dissolved in DMSO and incubated overnight at room temperature in the dark. Pass the above product through a diethylaminoethyl-Tris anion exchange column, and use NH 4 HCO 3 Gradient elution. Combine excess cholesterol chloroformate with folic acid-PEG-NH 2Dissolve in chloroform and react overnight at room temperature according to the above reaction conditions, and detect the progress of the reaction by ninhydrin reaction. The reaction product was dried under vacuum, washed with ether to remove unreacted cholesterol chloroformate, and the purity of the product was analyzed throu...
Embodiment 3
[0067] Example 3: Synthesis of Glycyrrhetinic Acid-PEG-Tocopherol Derivatives and Construction and Preparation of Self-Assembled Carriers and Biological Research
[0068] Appropriate amount of α-tocopheryl succinate was dissolved in dichloromethane, and functional HO-PEG-OH, dimethylaminopyridine, N-(3-dimethylaminopropyl)-N'-ethylcarbodiethylene were added in sequence Amino acid salt was stirred and reacted at room temperature for 16h. The product was separated and purified by silica gel column. Glycyrrhetinic acid is chemically reacted with PEGylated-tocopherol succinate to be connected through an ester bond. 1H-NMR (CDCl3) characterizes the synthesis of the product, δ5.69 and 5.6 are the chemical shifts of the hydrogen atoms of the glycyrrhetinic acid olefinic bond (-(C=O)-CH=C-), and δ3.52~3.76 are the chemical shifts of the PEG molecule Chemical shift, δ2.58~2.66 is the chemical shift of the succinic acid linking group, δ0.63 and 0.83~1.31 are the chemical shift of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com