HIV-1 genotype and drug resistant mutation site detection kit and application thereof
A drug-resistant mutation site, HIV-1 technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc. Expensive testing kits and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Amplification and sequencing primers of the Pol gene region of embodiment 1, HIV-1
[0089] The present invention downloads the full-length genome sequence of HIV-1 virus worldwide from the HIV database (http: / / www.hiv.lanl.gov / ), and utilizes appropriate evolutionary analysis software to analyze the global, Asian and Chinese The evolutionary analysis of the full-length genome sequence of the HIV-1 virus in China was carried out, and the evolutionary relationship and trend of different subtypes of HIV-1 viruses were obtained. According to the evolutionary relationship, the full-length genome sequences of 13 subtypes, including B, B' (Thailand B), CRF-01AE, CRF-07BC, and CRF-08BC, with high prevalence and wide geographical coverage were selected, and the appropriate nucleic acid homology analysis was used The software carries out conservation analysis to the Pol gene region of HIV-1, finds out the highly conserved section of the candidate (conservation is greater than 98...
Embodiment 2
[0102] Embodiment 2, HIV-1 genotype drug resistance detection kit and detection method thereof
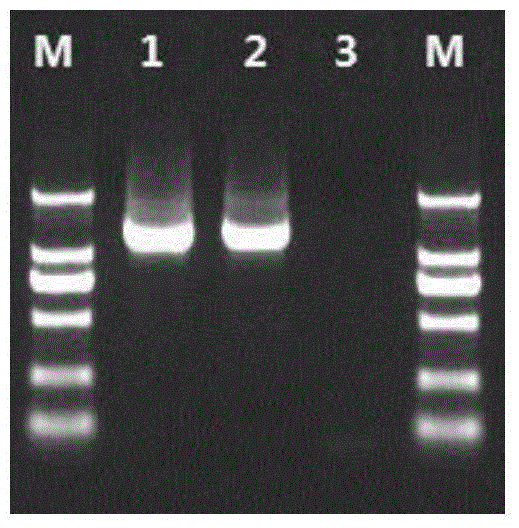
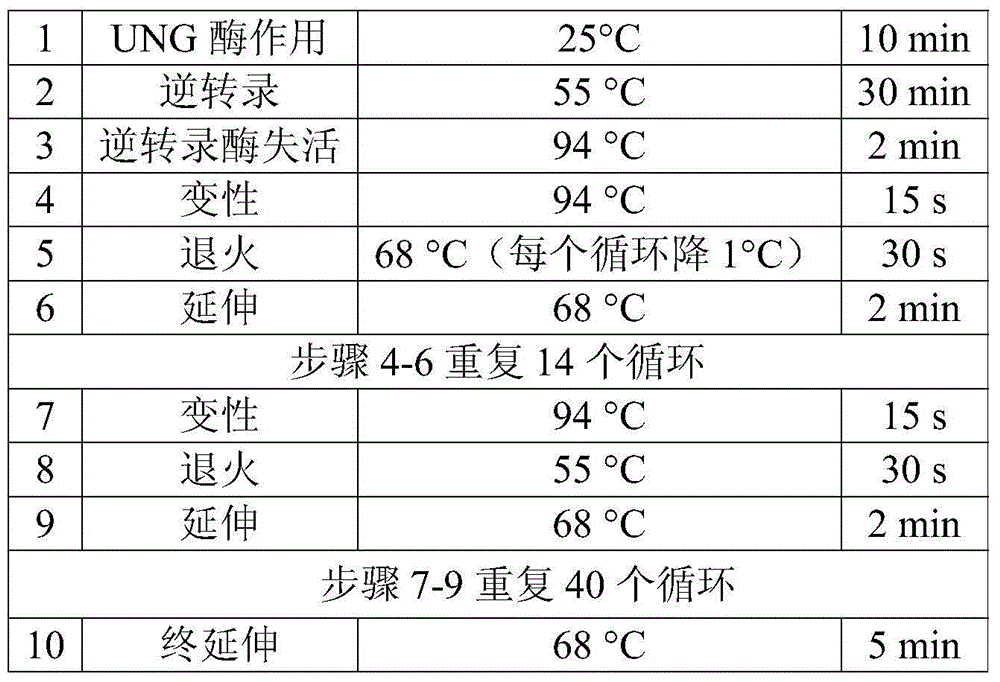
[0103] The kit of the present invention comprises RT-PCR amplification reagent A, RT-PCR amplification reagent B containing primer 1 and primer 2 in Example 1, RT-PCR amplification reagent C, and primer 3 in Example 1 PCR amplification reagent D with primer 8, sequencing amplification reagent 1-6 containing primer 3-primer 8 in Example 1. The detection method of the kit is as follows:
[0104] 1. Extraction of RNA
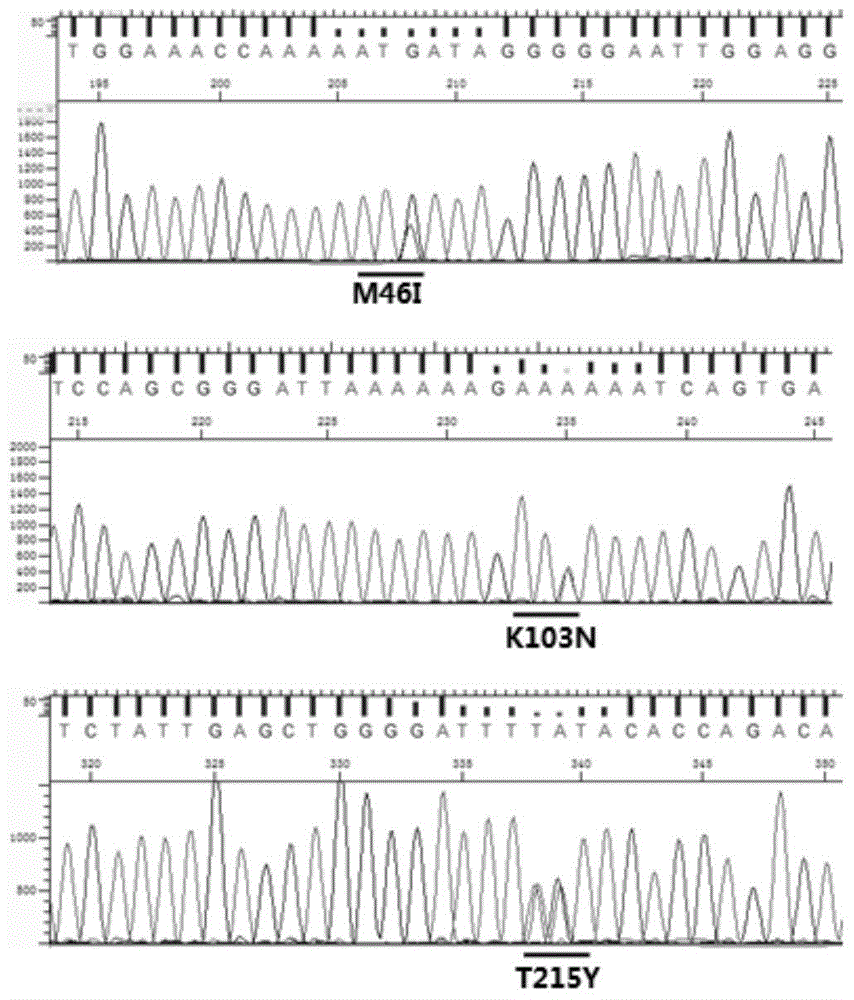
[0105] Extract the RNA in HIV-1 from the serum or plasma of the HIV-1 patient to be tested (from the AIDS Research Institute of the First Affiliated Hospital of China Medical University, informed by the patient, clinically diagnosed, containing drug resistance sites I84V, A71V and K101E) ,Specific steps are as follows:
[0106] 1. Add 20 μL proteinase K to a clean 1.5mL centrifuge tube;
[0107] 2. Add 200 μL of patient plasma sample to the centrifuge tube;
[0108...
Embodiment 3
[0186] Embodiment 3, the application of the detection kit of HIV-1 genotype drug resistance
[0187] Plasma samples of 50 HIV-1 infected persons with known genotypes and drug-resistant loci were collected (the First Affiliated Hospital of China Medical University, patients were informed and clinically diagnosed, and the genotypes and drug-resistant loci of 50 clinical samples were collected. Detecting result is shown in table 9), with the detection kit of HIV-1 genotype drug resistance and detection method thereof among the embodiment 2 to the genotype of 50 routine HIV-1 infected persons plasma samples and drug-resistant locus detection.
[0188] The genotype drug resistance detection results of 50 cases of clinical samples are as shown in Table 9: in 50 cases of clinical samples, the proportion of samples with drug resistance sites in the protease gene region was 22% (11 / 50); there was drug resistance in the reverse transcriptase gene region The sample ratio of the site is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com