Anti-human CEACAM5 monoclonal antibody and preparation method and application thereof
A technology of monoclonal antibody and deposit number, applied in the field of anti-human CEACAM5 monoclonal antibody and its preparation, can solve the problems of lack of antibodies and inability to apply molecules, and achieve the effect of high antigen specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 This example relates to the preparation of mouse anti-human CEACAM5 monoclonal antibody
[0050] The preparation method of mAb refers to the method described in "Cell and Molecular Immunology Experimental Technology", the first edition of Kim Boquan Fourth Military Medical University Press 2002, P9-P17, and the steps are as follows:
[0051] (1) Lyse human gastric cancer cell MKN-46-9, extract the whole cell protein of the lysate;
[0052] (2) Use the whole cell protein obtained in step (1) to immunize BALB / c mice at a dose of 50μg / mouse for 3 times; use Freund's complete adjuvant for the first immunization, and use Freund's incomplete adjuvant for the second immunization. The interval is 4 weeks, all are subcutaneous multiple injections, the third immunization is intraperitoneal injection, and the second immunization is 2 weeks apart;
[0053] (3) Step (2) Collect blood 7-10 days after immunization, and detect the titer by indirect ELISA; Inject immunogen into the ab...
Embodiment 2
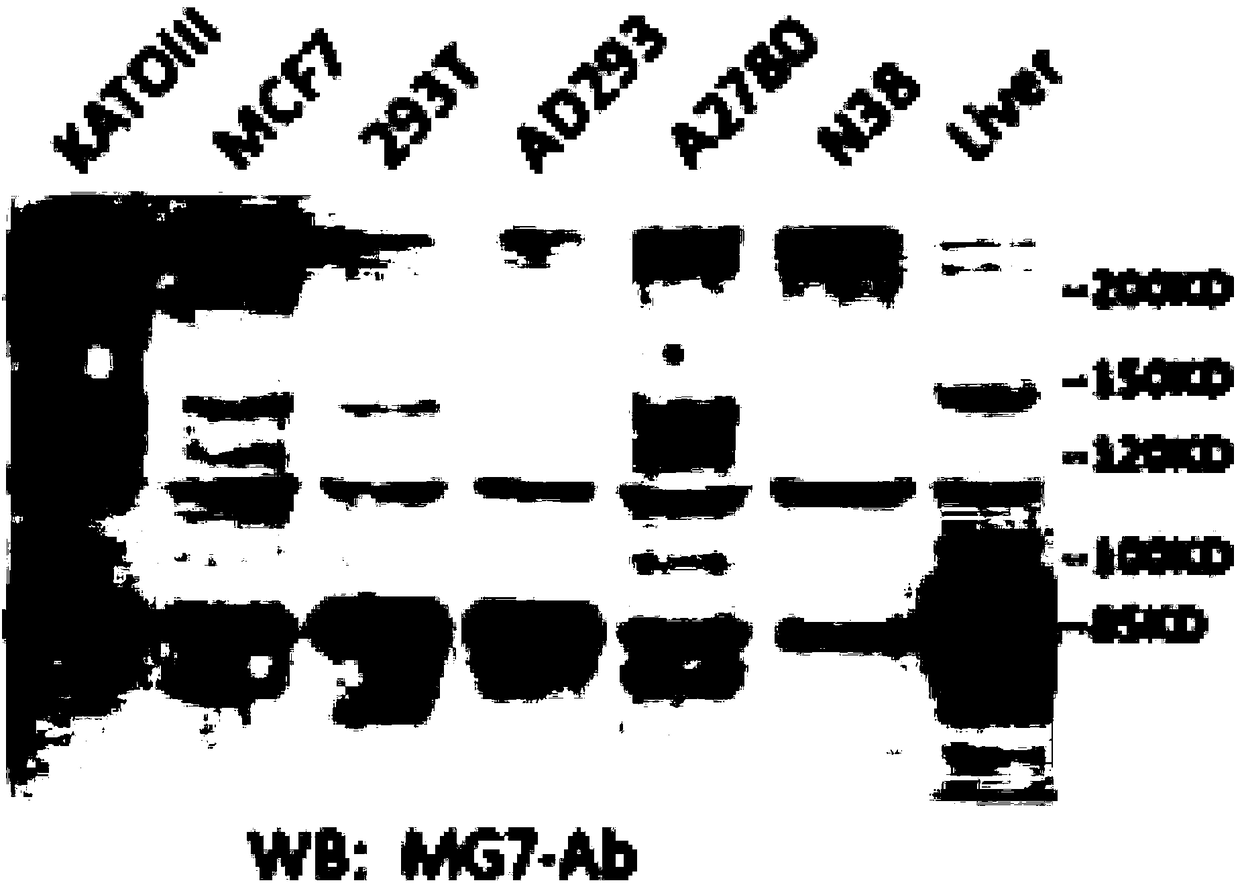
[0058] Example 2 This example relates to the Western blot detection of mouse anti-human CEACAM5 monoclonal antibody
[0059] Western blotting method was used to detect the binding of mouse anti-human CEACAM5 monoclonal antibody to gastric cancer cell line SGC7901, KATOIII cell lysate and non-gastric cancer cell line MCF7, HEK293T, AD293, A2780, N38 cell lysate and human liver tissue lysate. Ability, methods refer to the method described in "Cell and Molecular Immunology Experimental Technology", the first edition of Jin Boquan Fourth Military Medical University Press 2002, P53-P55, using mouse anti-human CEACAM5 monoclonal antibody as the primary antibody, and HRP-labeled goat anti The mouse antibody is a secondary antibody. The Alpha Innotech FluorChem FC2 imaging system (AlphaInnotech, USA) analyzes the results of Western hybridization.
[0060] Such as figure 1 As shown, the mouse anti-human CEACAM5 monoclonal antibody MG7-Ab of the present invention can react with the lysate of...
Embodiment 3
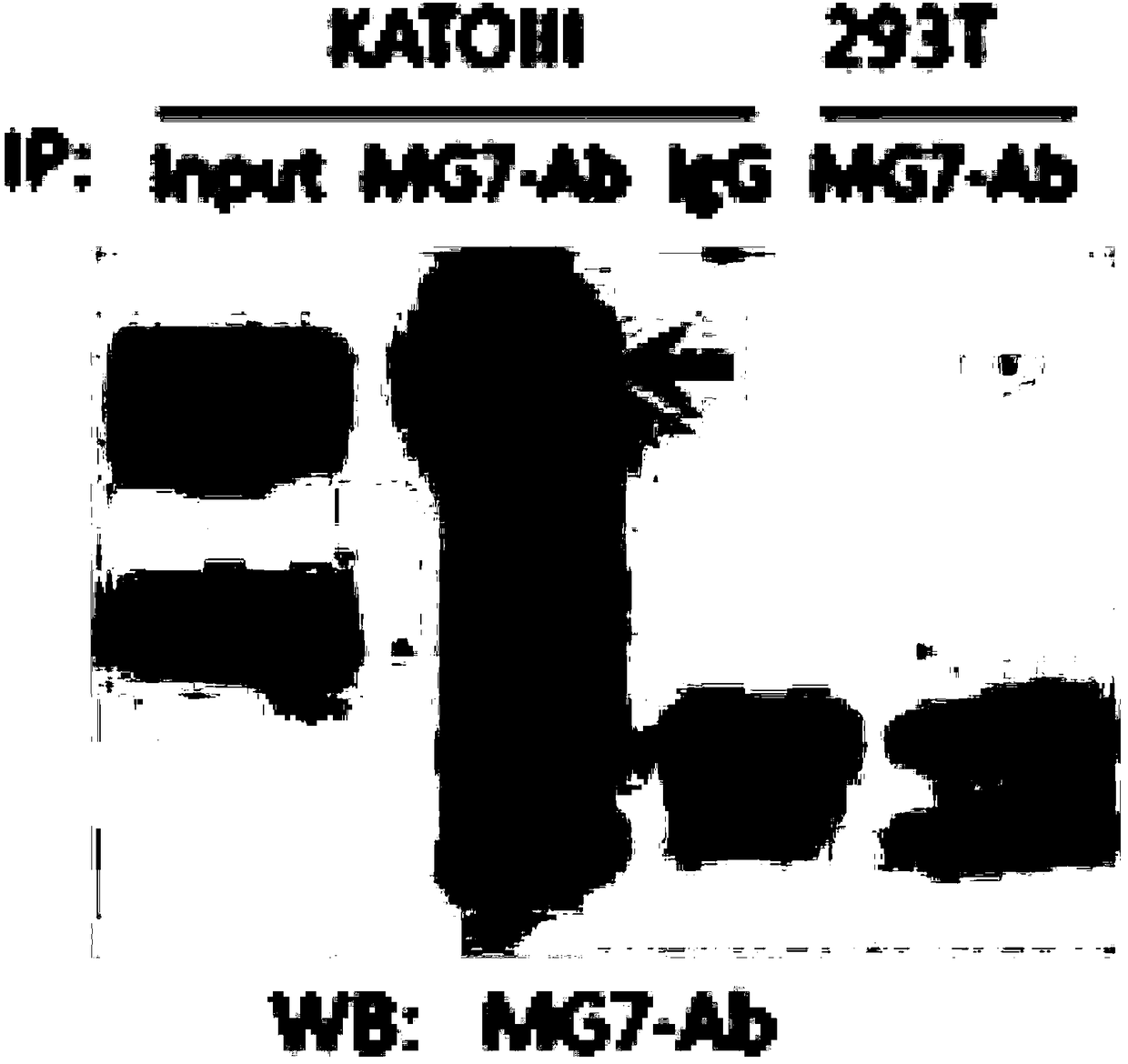
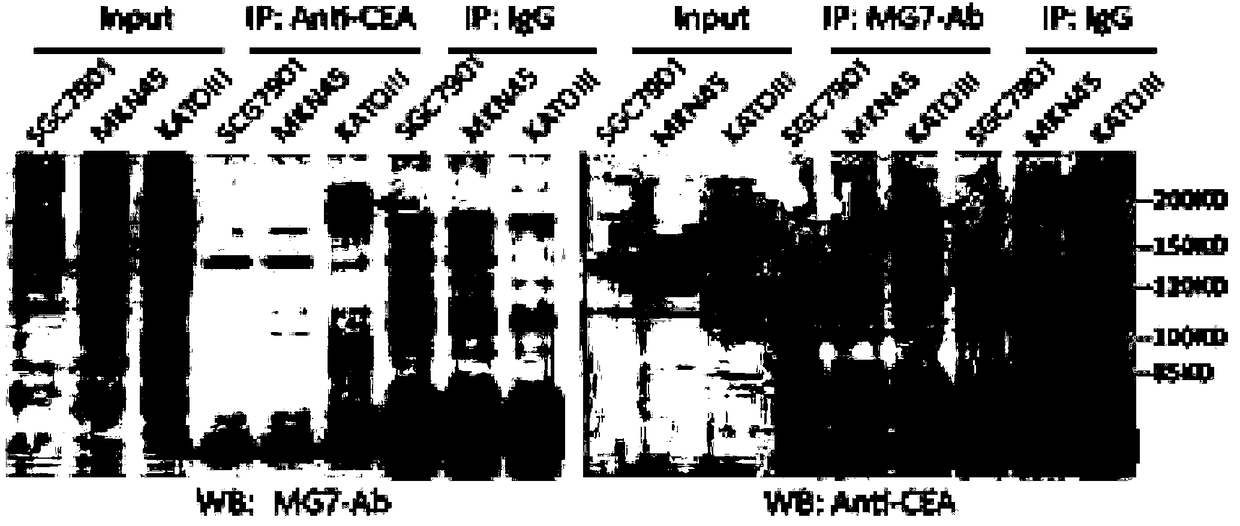
[0061] Example 3 This example relates to the identification of binding antigen of mouse anti-human CEACAM5 monoclonal antibody
[0062] The immunoprecipitation (IP) method was used to identify the antigen bound by the antibody MG7-Ab. The steps of IP are as follows: 1) Conventionally culture KATOIII cells on a 10cm culture plate to 90% confluence, and add 1ml IP Lysis Buffer (Pierce IP LysisBuffer, Prod #87788, containing protease inhibitor Roche cOmplete Tablets Mini, EDTA-free), lyse on ice for 30 minutes, 13,000×g, centrifuge at 4°C for 10 minutes and then take the supernatant. 2) Take a small amount of lysate for Western blot analysis, add protein A / G agarose beads (20mg / ml) to the remaining lysate for pre-clearing, and incubate with rotation at 4°C for 2 hours. 3) Centrifuge at 1000×g for 1 minute at 4°C and take the supernatant. This is the pre-cleared cell IP lysate. 4) Put 40mg protein A / G agarose beads into a 1ml Ep tube and wash them with PBS three times, then resuspen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com