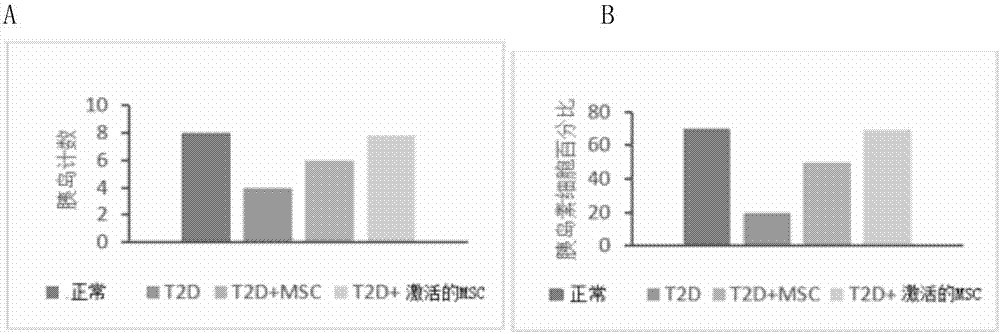
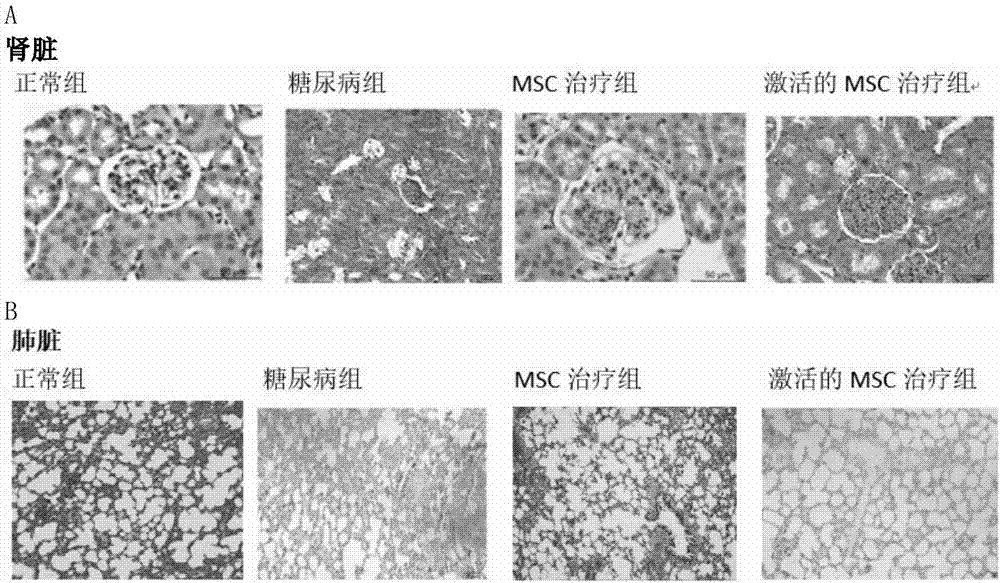
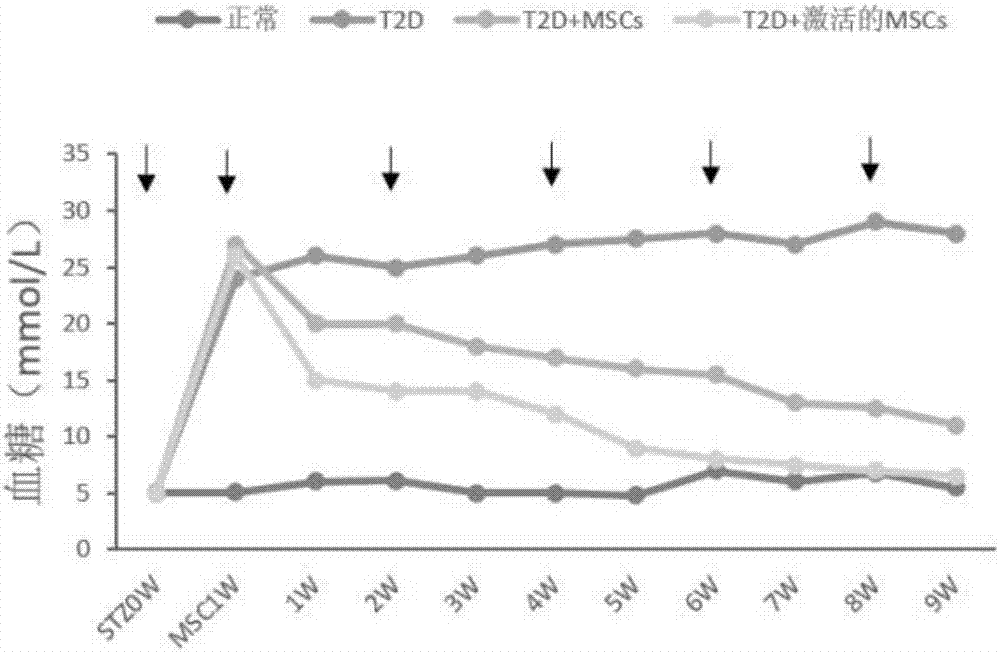
Active mesenchymal stem cell injection for diabetes mellitus infusion
A mesenchymal stem cell and diabetes technology, which is applied in the application of diabetes drugs and the field of active mesenchymal stem cell injection, can solve the problems of low efficiency and achieve the effects of improving efficiency, improving symptoms of kidney and lung complications, and easy quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1. Preparation of mesenchymal stem cells
[0033] 1. Preparation of umbilical cord mesenchymal stem cells
[0034] Operate in an ultra-clean workbench, take fresh full-term healthy fetal umbilical cord, wash it with normal saline containing 2 times 100U / mL penicillin and streptomycin to remove blood stains, peel off Walton’s jelly, and cut it into pieces less than 1 cubic millimeter Shape, spread evenly, slowly add 10mL serum-free medium, and culture in a 37°C, 5% carbon dioxide, saturated humidity incubator. After the primary cells were cultured for 7 days, the serum-free medium was replaced and cultured until the cells reached 70% confluence, the serum-free medium was removed, and trypsin-sodium edetate ( EDTA) for 1 min, the umbilical cord MSCs shrank and detached from the bottle wall. At this time, the previously removed culture supernatant was added to neutralize the trypsin-EDTA solution, and gently blown with a pipette, and the umbilical cord MSC suspens...
Embodiment 2
[0041] Example 2. Preparation of mesenchymal stem cells activated by diabetes-like microenvironment
[0042] The mesenchymal stem cells activated by the diabetes-like microenvironment are added with a certain concentration of glucose solution and palmitic acid solution. After culturing for a certain period of time, measure the number of cultured mesenchymal stem cells. If the concentration of mesenchymal stem cells can reach 5×10 5 ~2×10 6 cells / mL, indicating that glucose and palmitic acid solution have successfully activated mesenchymal stem cells. The concentration of palmitic acid is 0.2-0.4mmol / L; the concentration of glucose solution is 20-50mmol / L.
[0043] 1. Preparation of umbilical cord mesenchymal stem cells activated by diabetes-like microenvironment
[0044] (1)
[0045] When the umbilical cord MSC prepared in Example 1 is cultured to 40-50% monolayer cells in the fourth generation, glucose solution and palmitic acid solution are added, the final concentration ...
Embodiment 3
[0052] Example 3. Preparation of Mesenchymal Stem Cell Injection Activated by Diabetic Microenvironment
[0053] An injection of mesenchymal stem cells activated by a DM-like microenvironment, which includes the following components: the quantity is 5×10 5 ~2×10 6 DM-like microenvironment-activated mesenchymal stem cells / mL, the mass-volume ratio is 1-5% human serum albumin, the mass-volume ratio is 1-8% mannitol injection, and the mass-volume ratio is 1-5% compound The vitamin injection comprises a 50% glucose injection with a mass volume ratio of 1-5% and a balance of normal saline.
[0054]For example, prepare 100mL of DM-like microenvironment-activated mesenchymal stem cell injection, which includes 5mL of human serum albumin stock solution (final concentration of mass-volume ratio is 1%), 2mL of mannitol injection, 3mL of compound vitamin injection, 50% Glucose injection 5mL, saline 85mL, MSC activated by DM-like microenvironment. Except for the MSCs activated by the D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com