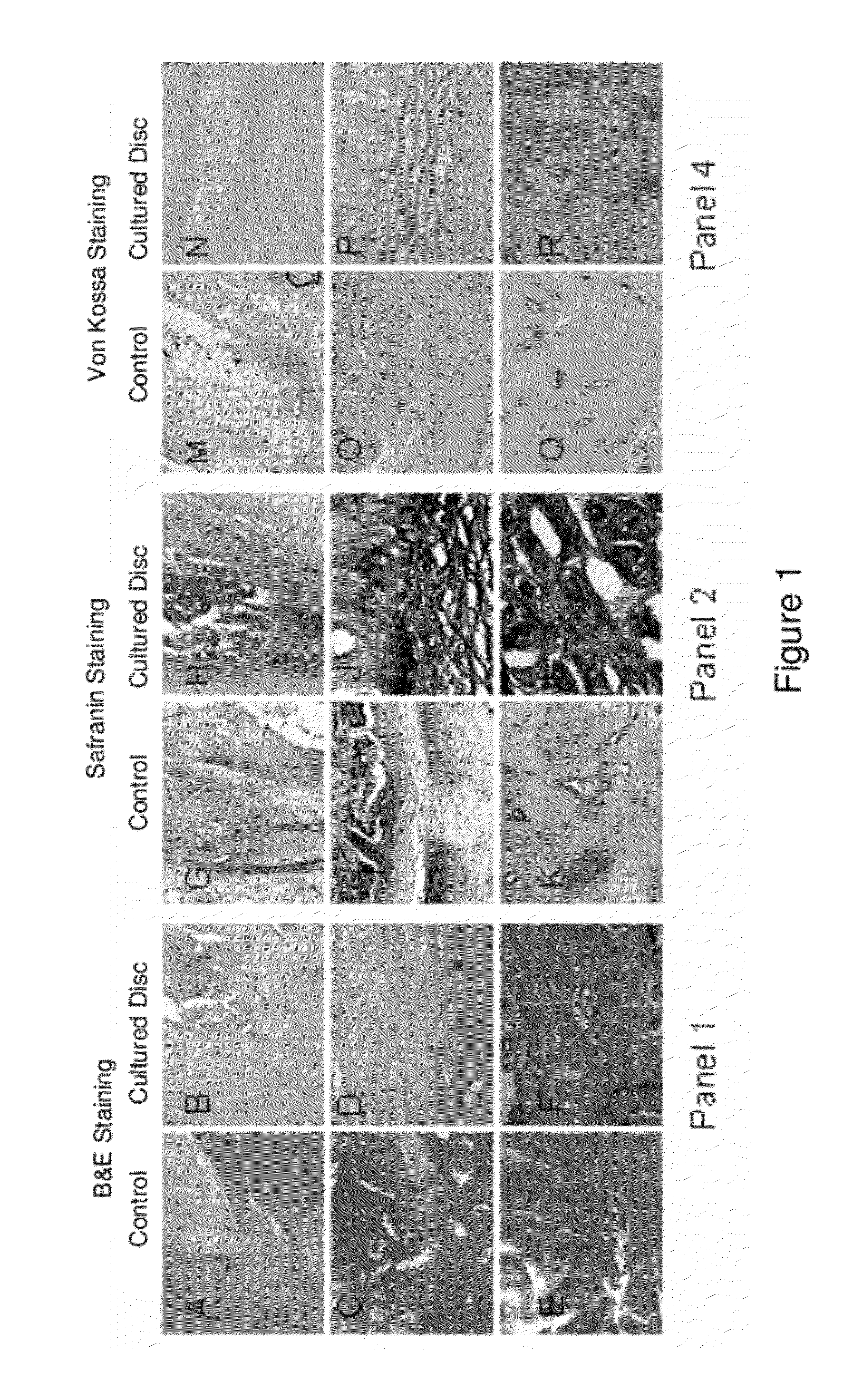
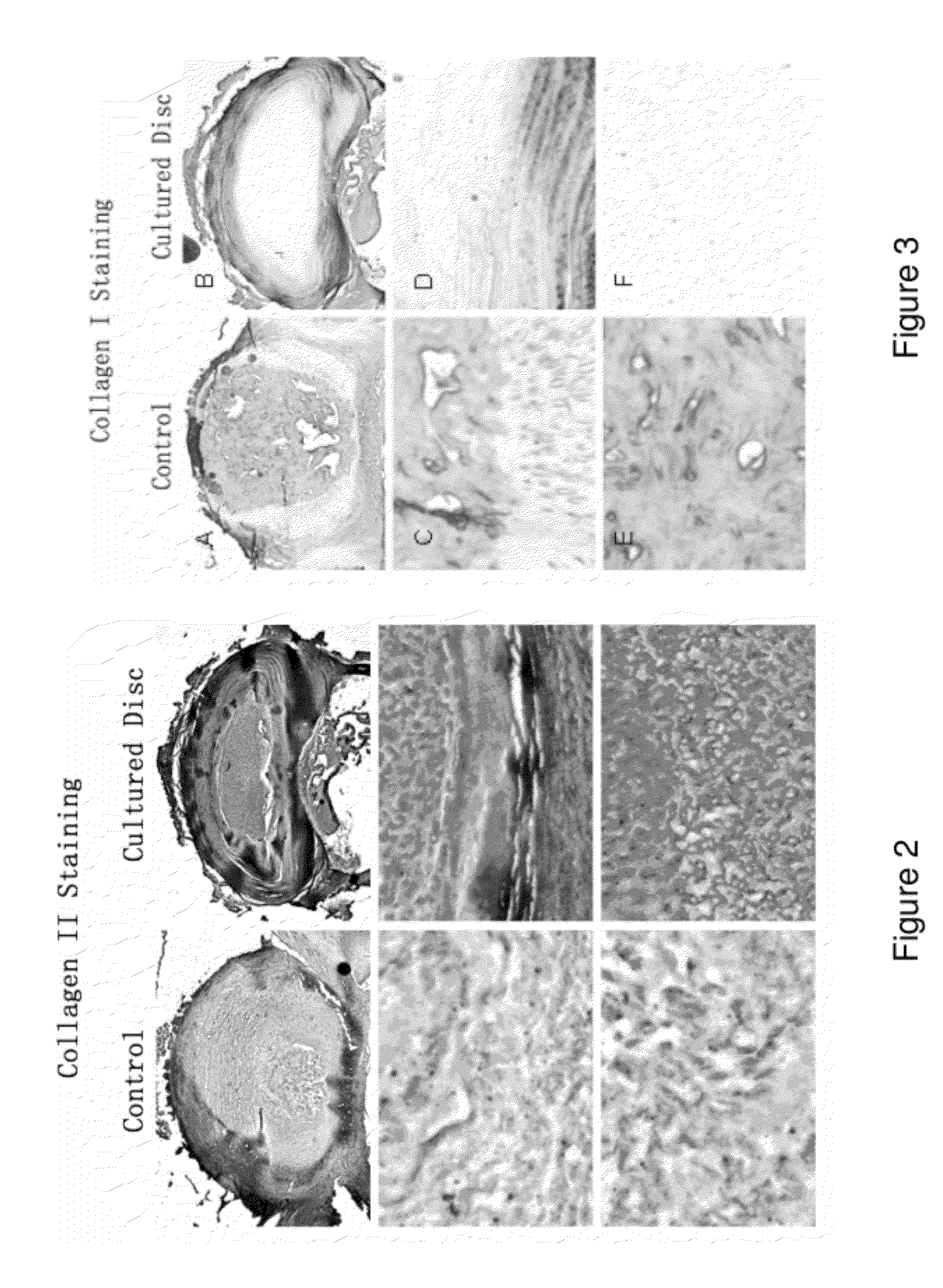
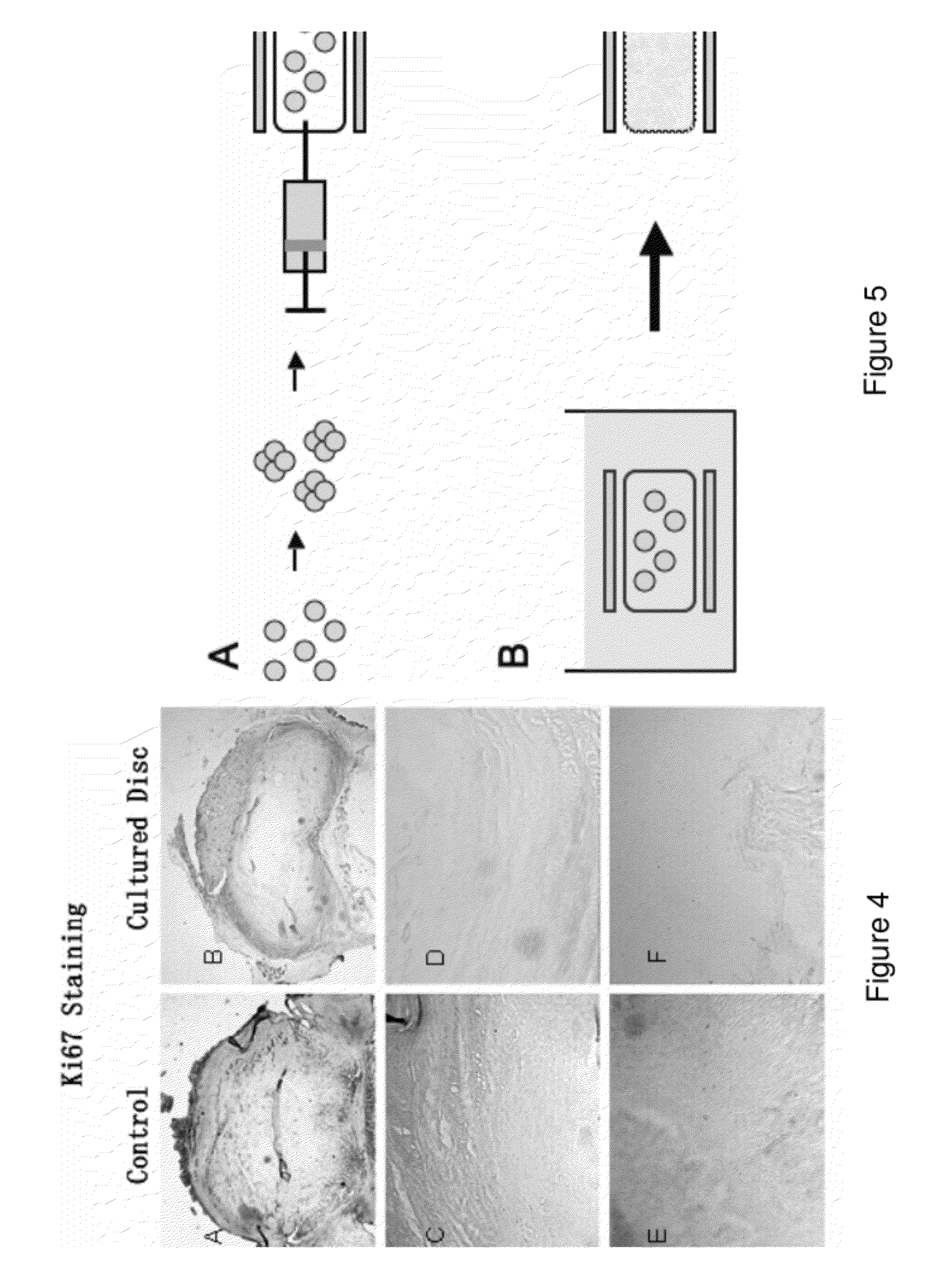
Compositions of adult disc stem cells and methods for the treatment of degenerative disc disease
a technology for disc disease and stem cells, applied in the field of adult disc stem cells and methods for the treatment of degenerative disc disease, can solve the problems of morbidity, disability, and productivity loss, and no treatment modalities have served as the “magic bullet” to eliminate or consistently improve this condition, and the prosthesis ultimately fails
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Method of Growing Discospheres
[0183]A biopsy specimen of human nucleus pulposus was minced into pieces approximately 2-3 millimeters in size and transferred to a 50 ml falcon tube containing 30 ml of Phosphate buffered saline (PBS) supplemented with standard antibiotics and antimycotics (standard penicillin / streptomycin solution (GIBCO BRL) in concentration 1:100).
[0184]PBS was aspirated and 30 ml of Dulbecco's Modified Eagle Media with F12 (DMEM / F12) medium containing 300 U / ml of Collagenase II solution was added to the 50 ml tube.
[0185]The tube was placed in a horizontal position in a shaker incubator at 37° C. at 100 RPM for 2-3 hours until fragments were completely dissociated.
[0186]The cell suspension was filtered through a nylon mesh into a 50 ml falcon tube and triturated with a fire-polished pasteur pipette to form a single-cell suspension. A cell count was performed at this point to determine the cell concentration.
[0187]The cell suspension was then centrifuged at room te...
example 2
A Method of Expanding Discospheres Cell Culture
[0194]Discospheres obtained by the method disclosed in Example 1 were dissociated by incubation at 37° C. in DMEM / F12 medium supplemented by collagenase II (300 U / ml).
[0195]Dissociated cells were expanded in 6-well plates according by passaging the cells using the same plating and culture techniques as described in Example 1.
example 3
A Method of Obtaining a Spinal Disc Collagen Scaffold (Annulus)
[0196]A postmortem (rabbit cadaver) intervertebral disc was removed by dissection with the vertebral endplates left intact. The intervertebral disc sample was soaked in 4 M guanidine thyocyonate for 24 hours at room temperature to remove intradisc biomaterial. After 24 hours, the intradisc biomaterial was liquidfied.
[0197]The liquid was aspirated, and the remaining disc scaffold was washed 3 times with room temperature PBS.
[0198]At this stage the disc scaffold can be stored in PBS at 4° C. up to one year.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com