Supplemented and unsupplemented tissue sealants, methods of their production and use
a technology of tissue sealant and supplement, which is applied in the direction of powder delivery, peptide/protein ingredients, and macromolecule non-active ingredients, etc. it can solve the problems of inconsistent use of fgf growth factor to promote wound healing, inability to establish if fgf growth factor is chemotactic for fibroblasts, and inability to possess true wound healing properties. , to achieve the effect of increasing the longevity and stability of fg, increasing stability, and increasing the shelf li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of HBGF-=1 for Supplementation of FG
[0249]An 800 ml culture of recombinant E. coli containing a plasmid that included DNA encoding HBGF-1β was prepared. After induction and culturing for 24 hours at 37° C., the cells were centrifuged and the supernatant was discarded. The cell pellet was resuspended in 25 mls of 20 mM phosphate buffer, containing 0.15 M NaCl, pH 7.3. The suspended cells were disrupted with a cell disrupter and the cell debris was separated from the resulting solution by centrifugation at 5000 g for 20 min.
[0250]The pellet was discarded and the supernatant containing the solubilized HBGF-1β and other bacterial proteins was loaded onto a 2.6 cm diameter by 10 cm high column of Heparin-Sepharose™ (Pharmacia Fine Chemicals, Upsala, Sweden). The column was washed with 5 column volumes of 0.15 M NaCl in 20 mM phsophate buffer, pH 7.3, and then was eluted with a 0.15 M NaCl in 20 mM phosphate buffer to 2.0 M NaCl gradient.
[0251]The eluate was monitored by UV ab...
example 2
Stability of HBGF-1
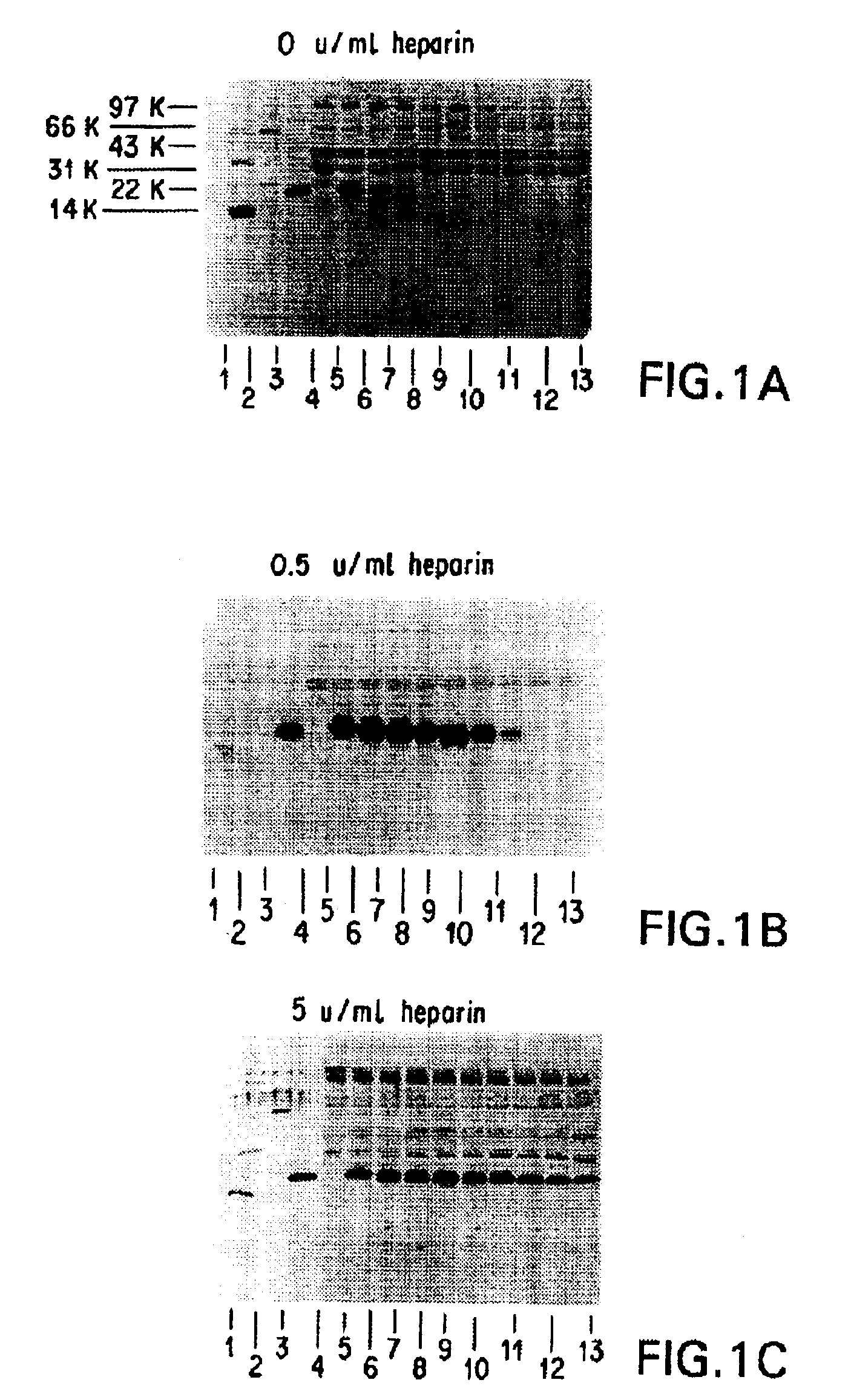
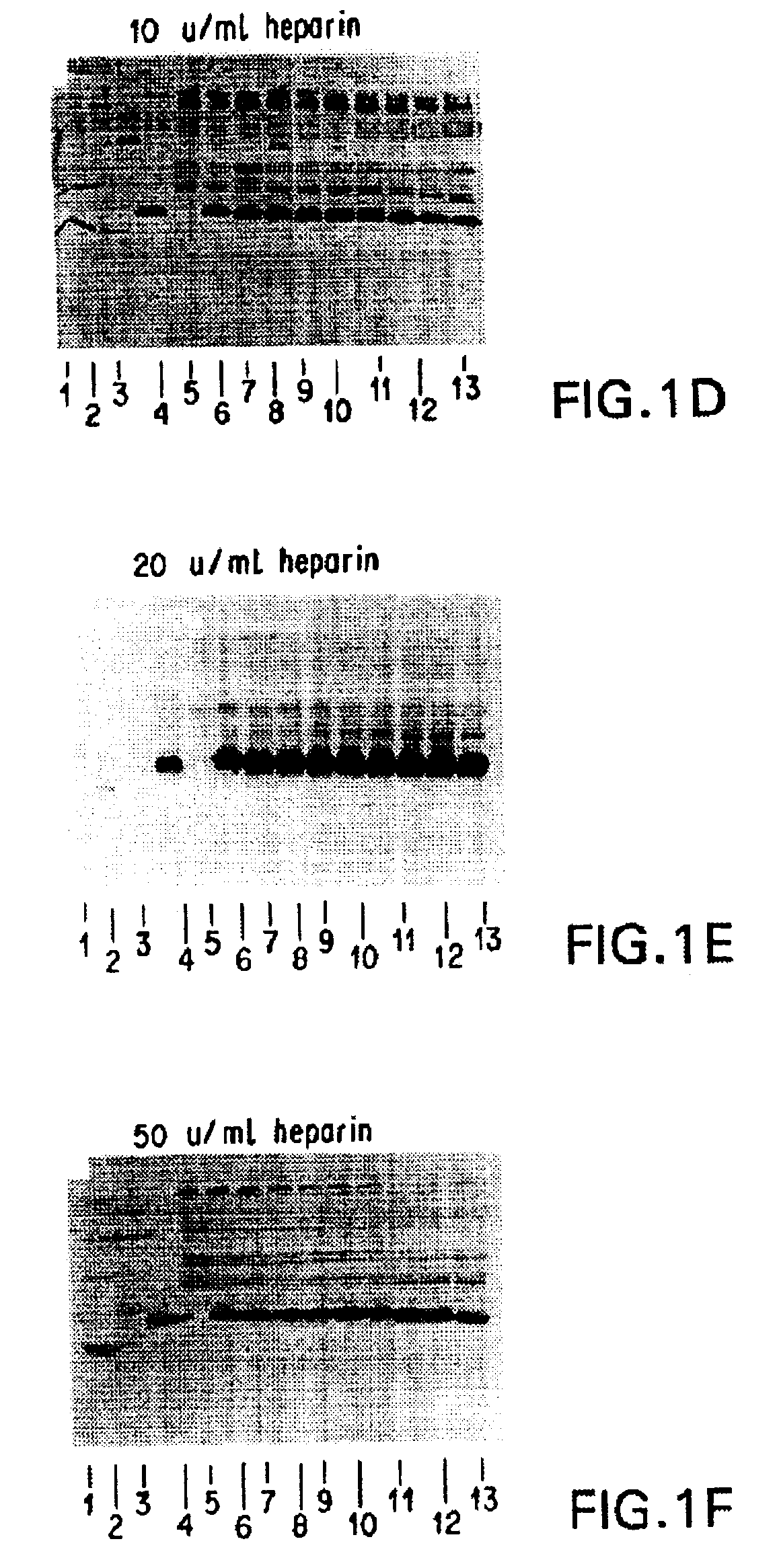
[0254]It was necessary to add an ingredient to the FG that would inhibit or prevent the digestion of HBGF-1β by thrombin (Lobb, Biochem. 27:2572-2578 (1988)), which is a component of FG. Heparin, which absorbs to HBGF-1, was selected and tested to determine whether it could protect HBGF-1 from digestion by thrombin and any other proteolytic components of the FG. The stability of HBGF-1 in the presence of increasing concentrations of heparin was assessed.
[0255]Solutions containing HBGF-1β (10 μg / ml), thrombin (250 U / ml), and increasing concentrations of heparin (0, 0.5, 5, 10, 20, and U / ml) were incubated at 37° C. Aliquots were periodically removed from the incubating solutions and were frozen and stored at −70° C. for further testing.
[0256]After the incubation was complete, the samples were thawed and separated on 15% SDS polyacrylamide gels under reducing conditions according to the method of Laemmli (Nature 227:680 (1970)). The gel was then electroblotted onto ...
example 3
The Biological Activity of HBGF-1β after Incubation in the Presence of Heparin and Thrombin
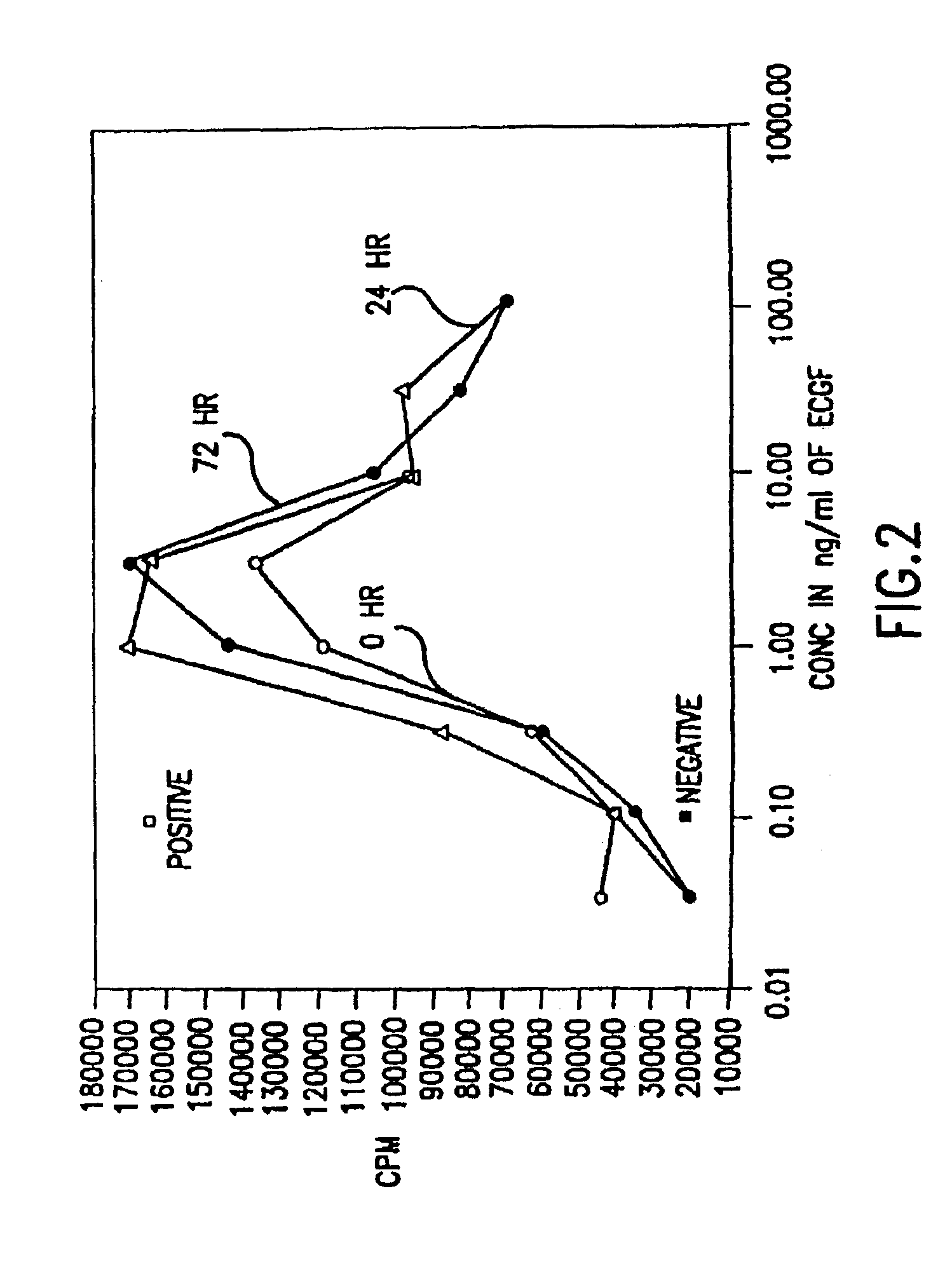
[0257]The biological activity of HBGF-1 in the incubation mixture that contained 5 U / ml of heparin, and was described in Example 2, was measuring using an 3H-thymidine incorporation assay with NIH 3T3 cells.
[0258]NIH 3T3 cells were introduced into 96 well plates and were incubated at 37° C. under starvation conditions in Dulbecco's Modified Medium (DMEM; GIBCO, Grand Island, N.Y.) with 0.5% et al bovine serum (BCS; GIBCO, Grand Island, N.Y.) until the cells reached 30 to 50% confluence. Two days later, varying dilutions of HBGF-1 from the samples prepared in Example 2 were added to each well without changing the medium. Diluent (incubation buffer) was added in place of growth factor for the negative controls and DMEM with 10% BCS, which contains growth factors needed for growth, was added in place of the HBGF-1 sample for the positive controls.
[0259]After incubation at 37° C. for 18 hours, 0.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com