Method for editing swine BMP15 (bone morphogenetic protein 15) gene by using CRISPR/Cas9
A gRNA-e1s1, gene technology, applied in the field of editing pig BMP15 gene using CRISPR/Cas9, can solve the problems of loss of reproductive ability of sheep, few research reports, and increase of sheep litter size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
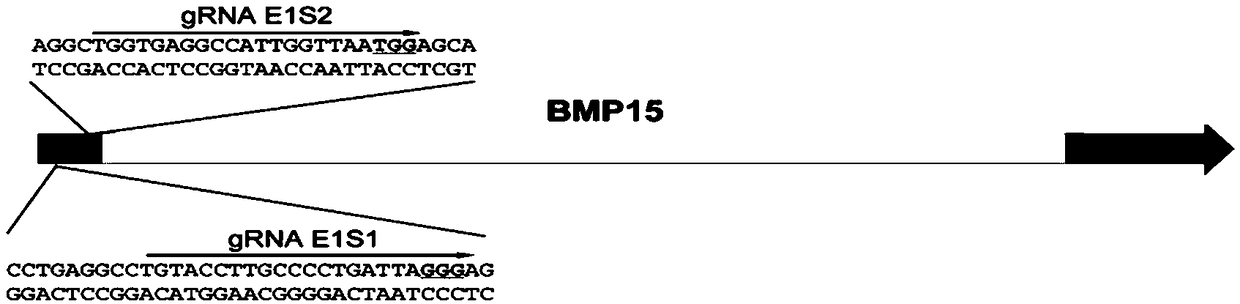
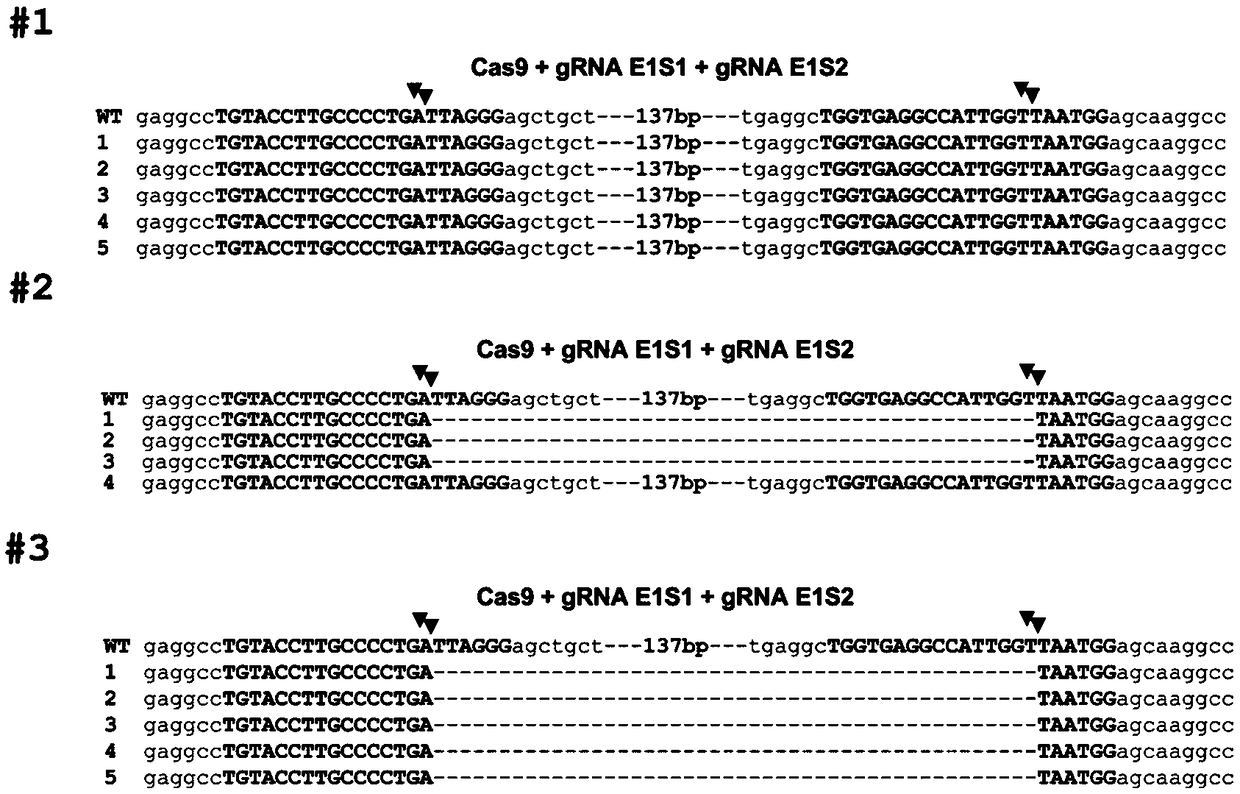
[0034] Using CRISPR / Cas9 to Edit Porcine BMP15 Gene to Prepare Related Gene Edited Cells
[0035] 1. Obtaining isolated porcine fetal kidney cells
[0036] Pig fetal kidney cells are isolated from large white pig fetal kidneys, and the pig fetal kidney cells are separated in an ultra-clean bench. Use scissors and tweezers to remove the kidney tissue of the fetus, wash the removed tissue repeatedly in 75% alcohol and PBS with antibiotics in sequence, cut the tissue piece to a size of 1 cubic millimeter with small scissors, and centrifuge at 1600rpm for 5 minutes to remove the PBS. Then add 20% FBS DMEM with antibiotics, gently pipette evenly, and place in a 37°C cell culture incubator for cultivation. After putting it into the cell culture box, do not move the culture dish. Three days later, it can be observed that the pig fetal kidney cells have covered the entire culture dish, and then the digestion and culture of ordinary passaged cells can be carried out.
[0037] 2. Obta...
Embodiment 2
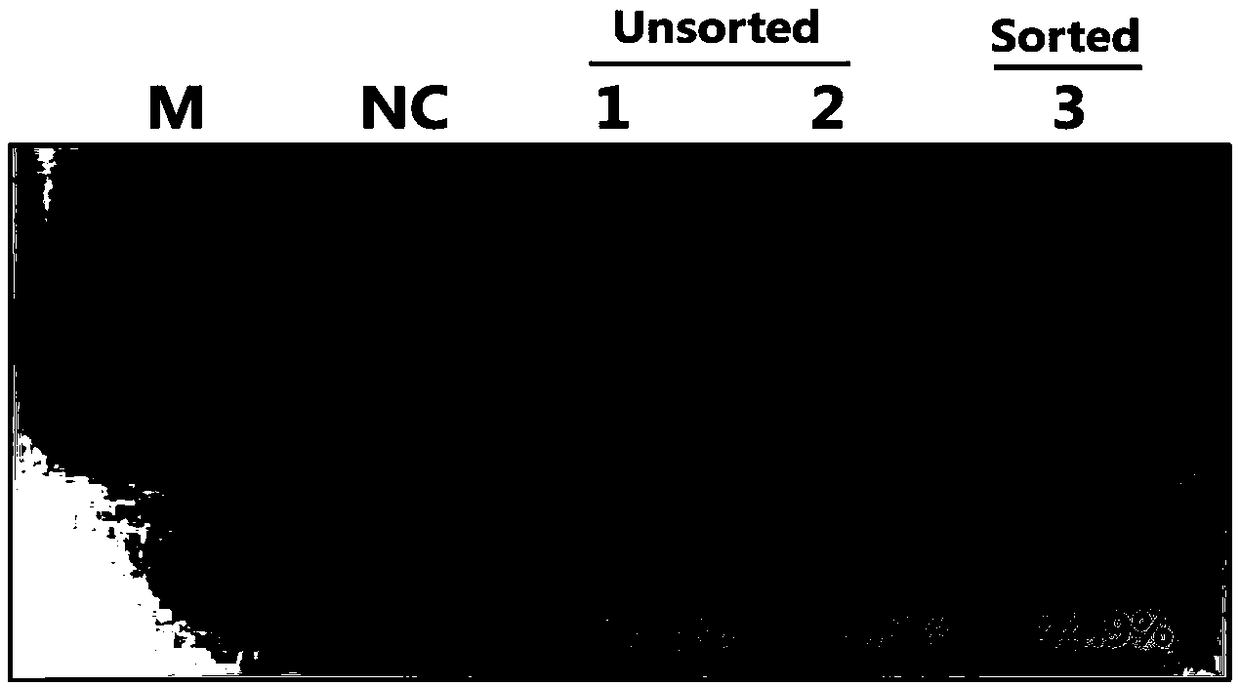
[0051] Example 2: Construction of BMP15 gene-edited pigs using somatic cell nuclear transfer technology
[0052] 1. Somatic cell nuclear transfer to obtain BMP15 gene-edited pigs
[0053] Select ovaries with appropriate developmental stages from healthy Large White sows, extract the contents of follicles with a diameter of 3-5 mm on the surface of the ovary with a syringe, dilute the contents in TL-PVA and resuspend to form a suspension. The suspension was left standing at 37°C until the oocytes were completely precipitated, and the oocytes were sucked out and placed under a stereoscope with a pipette or a suction pipette to pick out oocytes with complete pericytes. The selected healthy oocytes were put into TCM-199 containing 10% follicular fluid, FSH, LH, EGF and cultured for 22 hours. Then use a pipette or mouth pipette to move the oocytes to TCM-199 containing 10% follicular fluid and EGF and continue to culture for 22 hours. After 44 hours of culture and maturation, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com