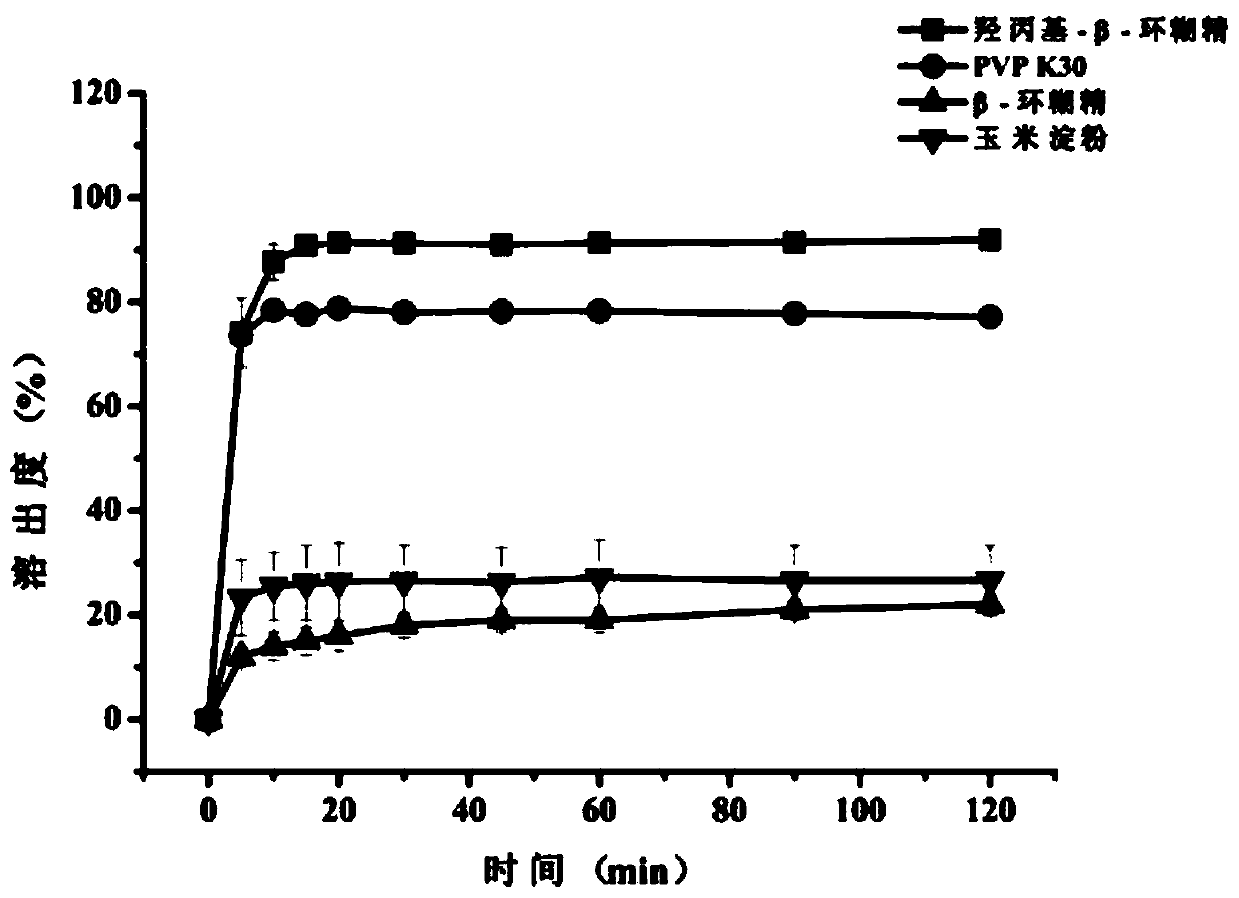
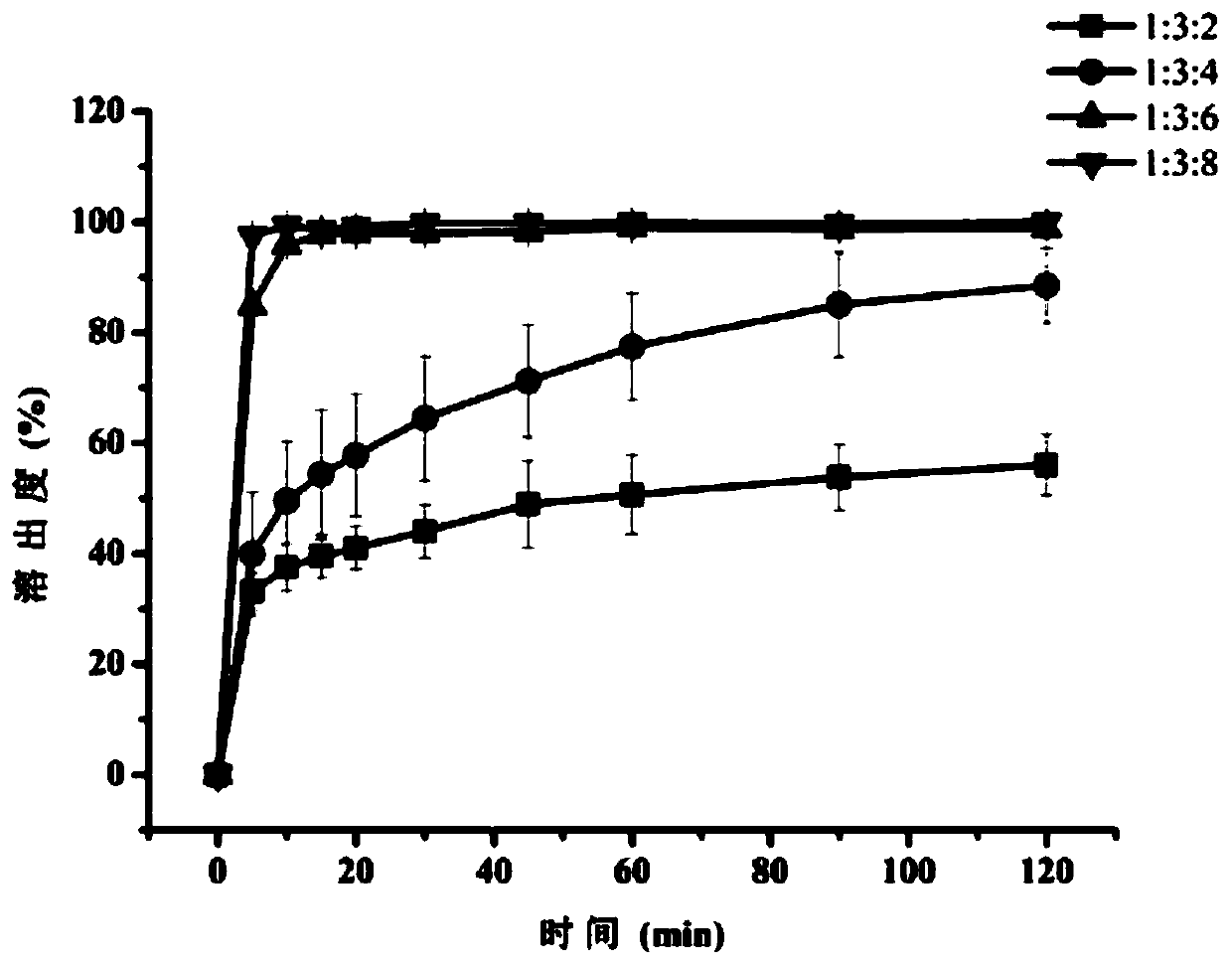
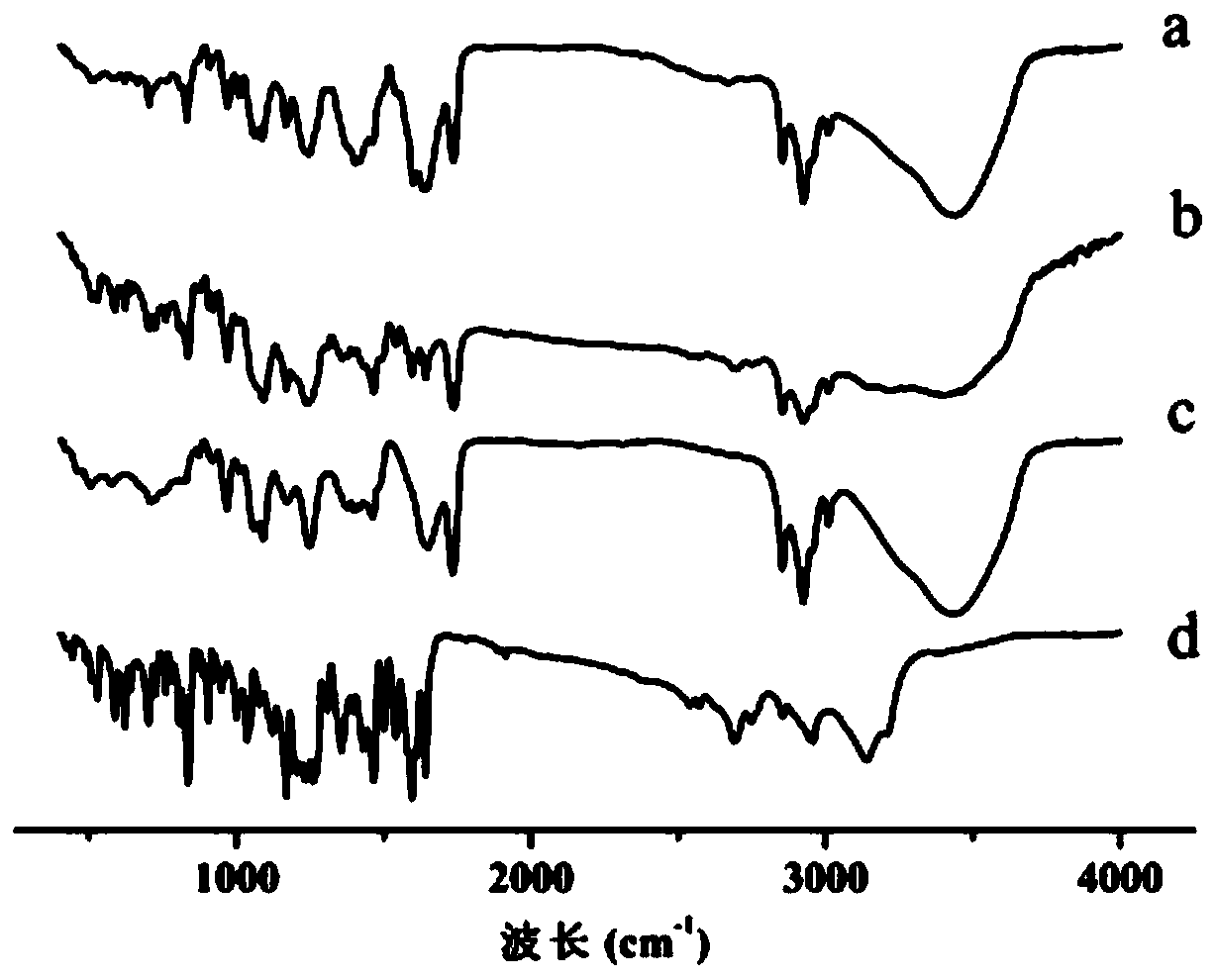
Raloxifene hydrochloride phospholipid complex solid dispersion and preparation thereof
A technology of raloxifene hydrochloride and phospholipid complex, which is applied in the field of medicine, and can solve the problems of undisclosed raloxifene hydrochloride phospholipid complex solid dispersion, different physical and chemical properties, and different solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0055]Example 1: The mass ratio of raloxifene hydrochloride to phospholipid is 1:3, and the reaction solvent is tetrahydrofuran.
[0056] Weigh 150 mg of raloxifene hydrochloride and 450 mg of phospholipids, and put them into a 100 mL eggplant-shaped flask. Add 30mL of tetrahydrofuran, so that the reaction concentration of the drug is 5mg / mL, and the temperature of the water bath is raised to 60°C. The system was maintained under this condition for 2 hours, the solvent was recovered, and dried to obtain the raloxifene hydrochloride phospholipid complex. The compound rate of the drug was determined to be 27.3% according to the compound rate determination method described below.
example 2
[0057] Example 2: The mass ratio of raloxifene hydrochloride to phospholipids is 1:3, and the reaction solvent is absolute ethanol.
[0058] Weigh 150 mg of raloxifene hydrochloride and 450 mg of phospholipids, and put them into a 100 mL eggplant-shaped flask. Add 30mL of absolute ethanol to make the reaction concentration of the drug 5mg / mL, and the temperature of the water bath is raised to 60°C. The system was maintained under this condition for 2 hours, the solvent was recovered, and dried to obtain the raloxifene hydrochloride phospholipid complex. The compounding rate of the drug was determined to be 71.9% according to the compounding rate determination method described below.
example 3
[0059] Example 3: The mass ratio of raloxifene hydrochloride to phospholipids is 1:3, and the reaction solvent is acetone.
[0060] Weigh 150 mg of raloxifene hydrochloride and 450 mg of phospholipids, and put them into a 100 mL eggplant-shaped flask. Add 30 mL of acetone to make the reaction concentration of the drug 5 mg / mL, and raise the temperature of the water bath to 60°C. The system was maintained under this condition for 2 hours, the solvent was recovered and dried to obtain the raloxifene hydrochloride phospholipid complex. The compound rate of the drug was determined to be 68.1% by the compound rate determination method described below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com