Raloxifene derivative and preparation method thereof
A technology of raloxifene and its derivatives, which is applied in the field of raloxifene derivatives and its preparation, can solve problems such as complex reaction raw materials, poor regioselectivity, and functional group tolerance and limitations, and achieve easy reaction conditions, The effect of simple reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
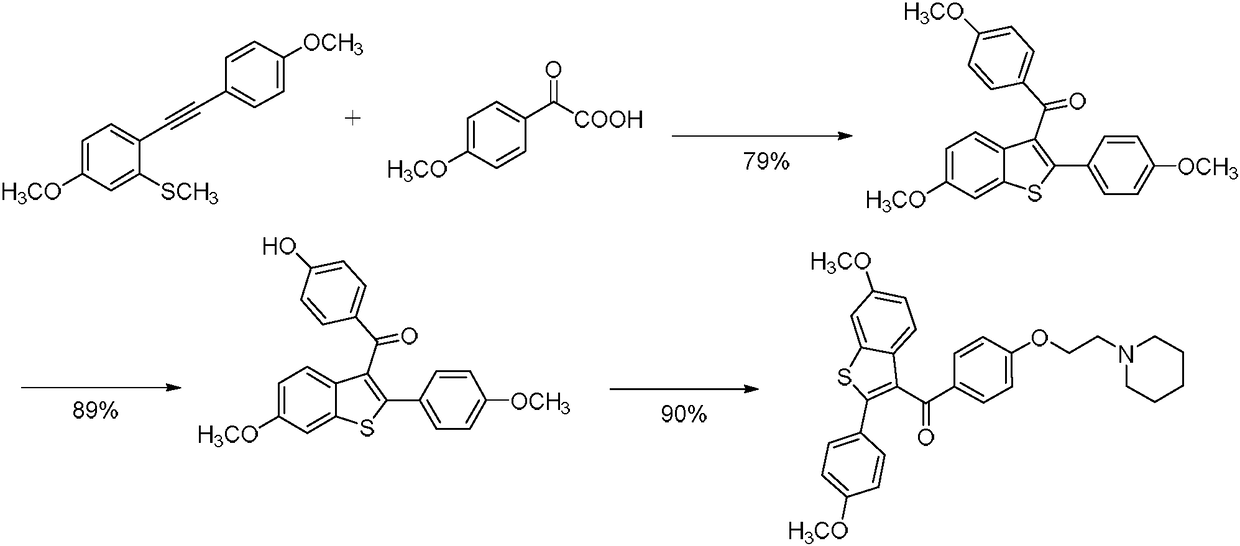
[0040] step one:
[0041] react compound I and compound II in a solvent under the action of an Ag catalyst and an oxidizing agent to obtain compound II;
[0042] The compound I structural formula is:
[0043] The compound II structural formula is:
[0044] The structural formula of the compound III is:
[0045] Step two:
[0046] Reaction of compound III with NaSEt in a solvent to obtain compound VI;
[0047] The structural formula of the compound VI is:
[0048] Step three:
[0049] Compound IV and 1-(2-chloroethyl)piperidine, CsCO 3 , KI react to obtain raloxifene derivatives;
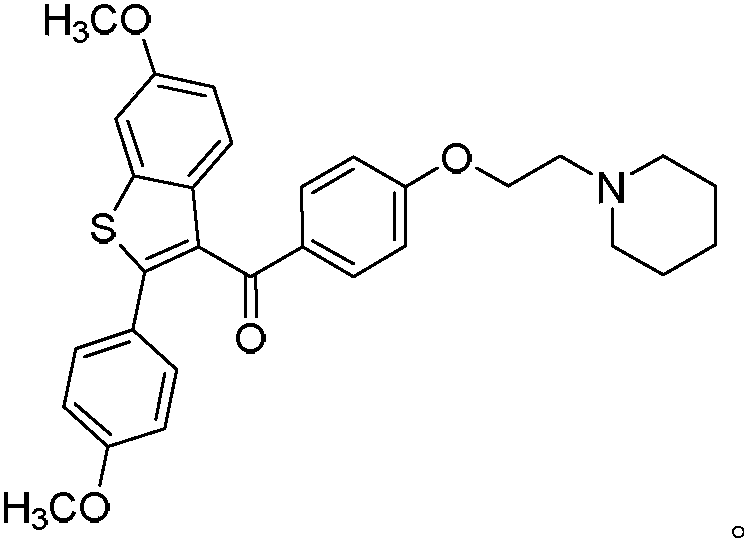
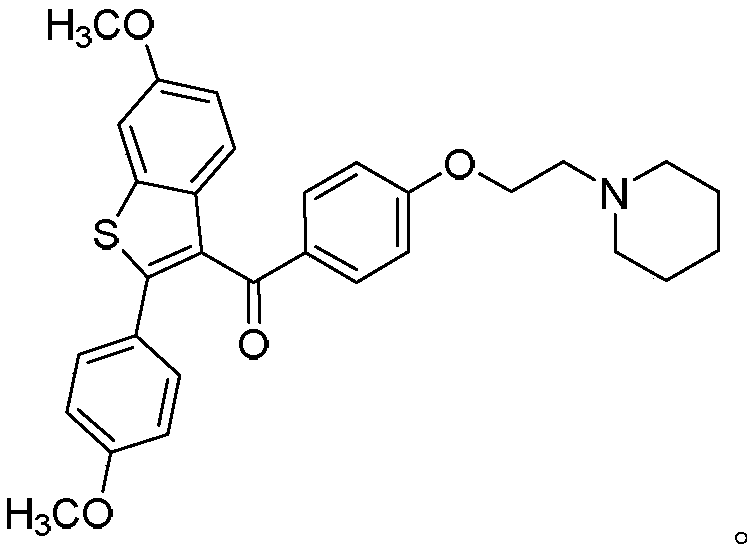
[0050] The structural formula of the raloxifene derivative is
[0051] The oxidant in the step 1 is K 2 S 2 o 8 .
[0052] The catalyst in said step one is silver nitrate.
[0053] The solvent of the step one is CH with a volume ratio of 1:1 3 CN and H 2 O mixture.
[0054] In the first step, the reaction is carried out under nitrogen atmosphere, the reaction temperature is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com