Method for chemoprevention of prostate cancer
A prostate cancer and application technology, applied in the direction of pharmaceutical formulations, non-active ingredients of polymer compounds, medical preparations containing active ingredients, etc., can solve the concerns of patients and doctors, and the lack of methods for treating patients with prostate intraepithelial tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation of pharmaceutical compositions containing active ingredients is known in the art. Typically, such compositions are prepared as an aerosol of the polypeptide for administration to the nasopharynx, or as injectable solutions or suspensions, however, solid forms suitable for solution in, or suspension in, liquid prior to injection can also be prepared. The formulation may be emulsified. The active therapeutic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. Suitable excipients are, for example, water, saline, dextrose, glycerol, ethanol, etc. and combinations thereof. In addition, the composition, if desired, can contain minor amounts of auxiliary substances such as wetting or emulsifying agents, pH buffering agents, which enhance the effectiveness of the active ingredients.
[0044] The active ingredients can be formulated into compositions in the form of neutral pharmaceutically...
Embodiment 1
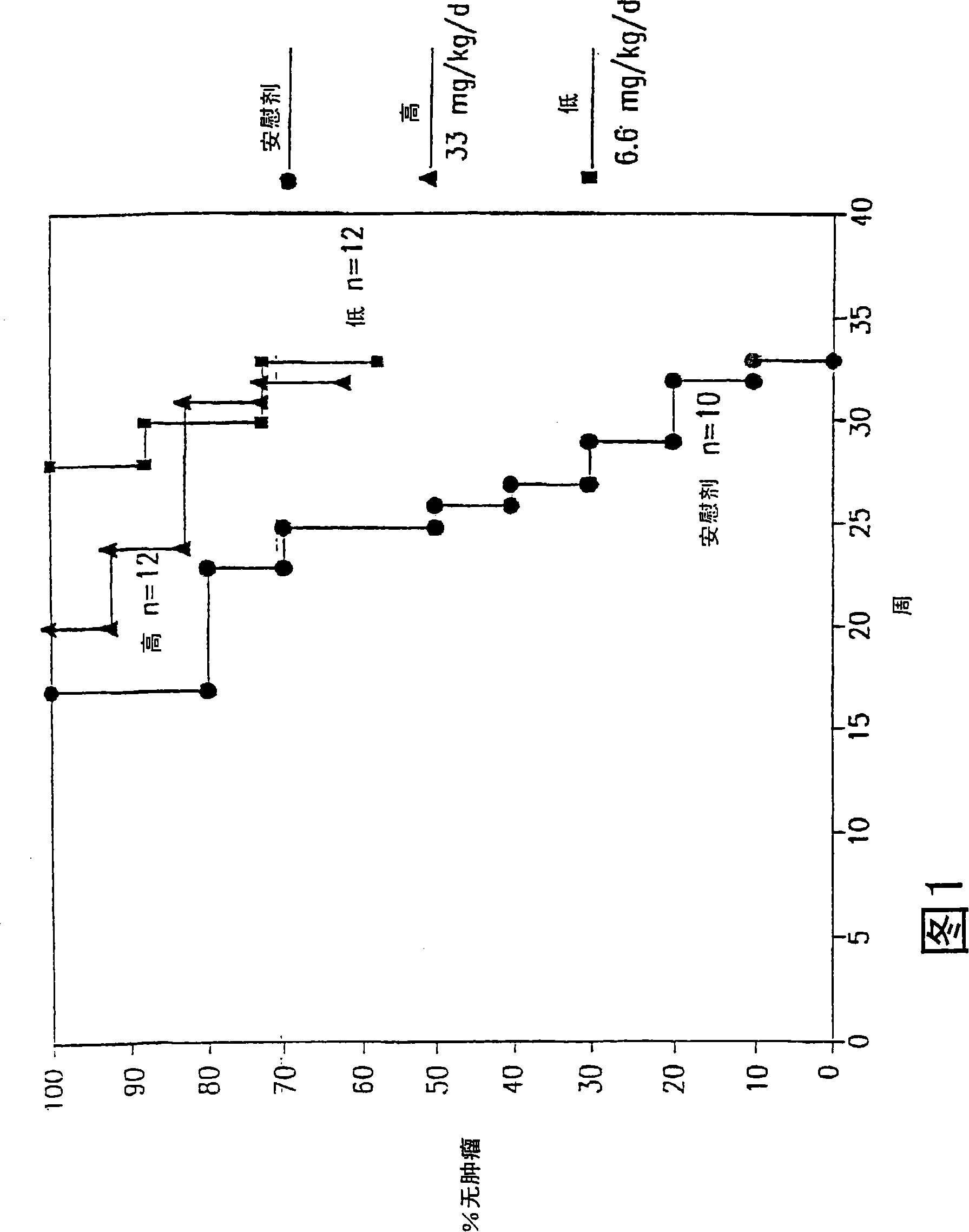
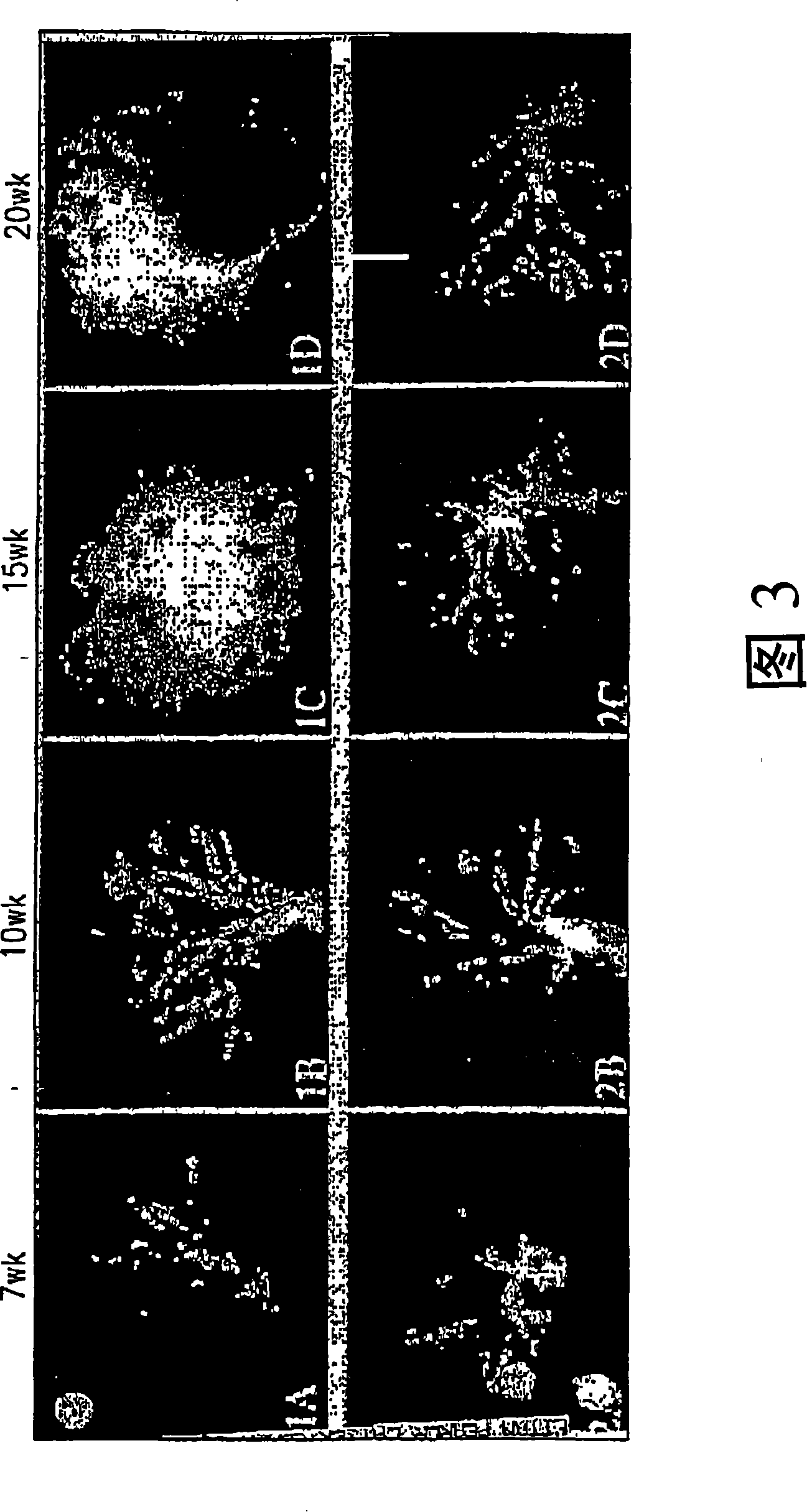
[0055] Example 1: Transgenic adenocarcinoma mouse prostate
[0056] Research on prostate cancer chemoprevention is hampered by a lack of suitable animal models. The recently developed transgenic adenocarcinoma mouse prostate (TRAMP) model has enabled the study of chemoprevention. In the TRAMP model described in Greenberg et al., Prostate cancer in a transgenic mouse, Proc.Natl Acad.Sci.USA, 1995, Vol.92, 3439-3443 pages, the PB-SV40 large T antigen (PB-Tag) transgene is in Specifically expressed in mouse prostate epithelial cells. The results show that this model has several advantages over existing models: 1) mice develop progressive prostatic epithelial hyperplasia at 10 weeks of age and invasive adenocarcinoma at about 18 weeks of age; 2) metastatic spread of prostate cancer The pattern mimics human prostate cancer, and the common metastatic sites are lymph nodes, lungs, kidneys, adrenal glands, and bones; 3) The occurrence and progression of prostate cancer can occur wit...
Embodiment 2
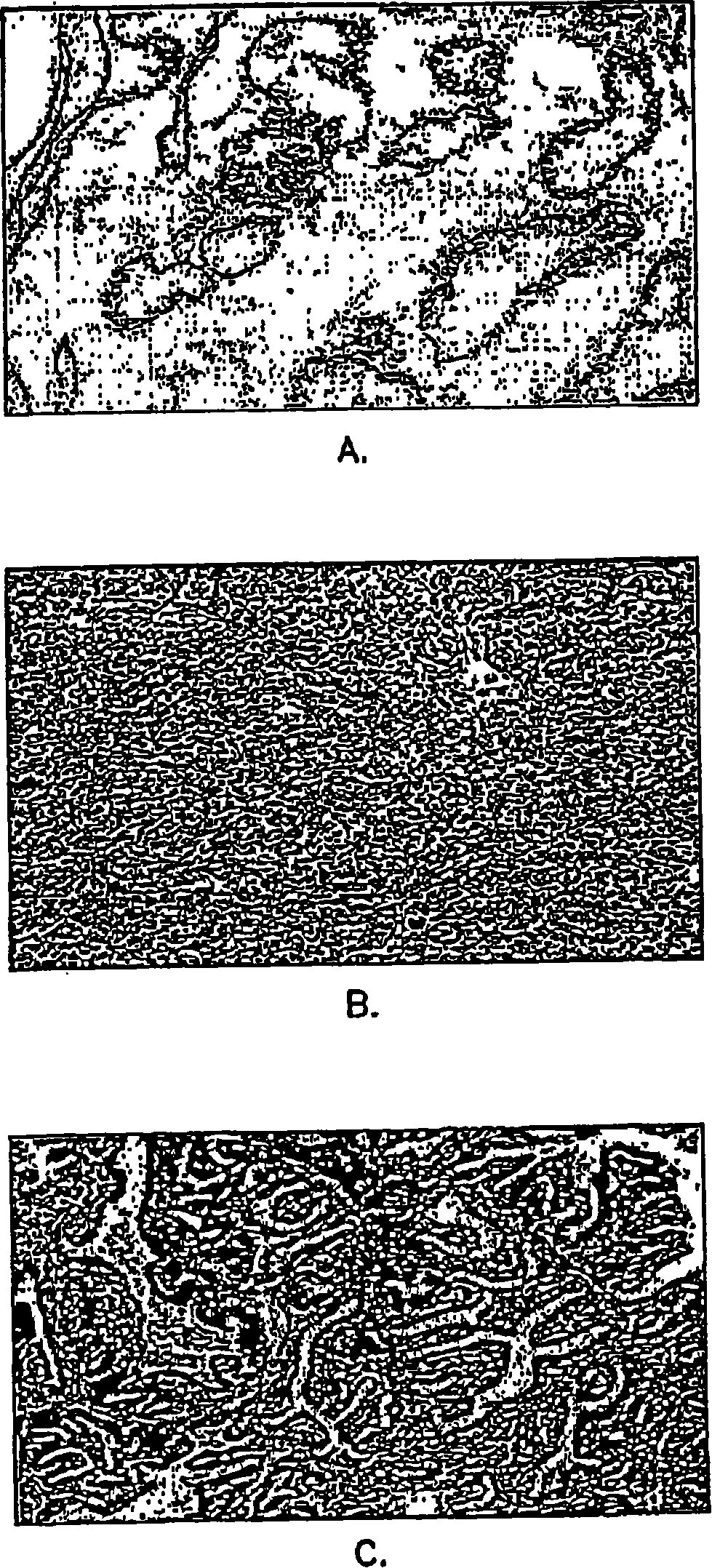
[0062] Example 2: Immunohistochemical data analysis
[0063] Microscopic images of each tissue section were evaluated by computer-aided (Mac 9500-I 32 computer and monitor) image quantification (NIH-Image 1.6PPC) with a Kodak DCS 460 camera on a Nikon Microphot-FX microscope, and were evaluated by using Quantification was obtained by color-assisted quantification system image analysis (IPLab Spectrum 3.1, Scanalytics, Inc., VA) that distinguishes color differences in stained tissue sections. Thresholds were set to identify various tissue components of the prostate. For each full screen of a color monitor, the area pixel density corresponding to each of these tissue components is calculated. A total of 5 screens per prostate section were averaged. Immunohistochemical images can be digitized and quantified, enabling statistical evaluation by determining correlation coefficients and probabilities (2-tailed) of samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com