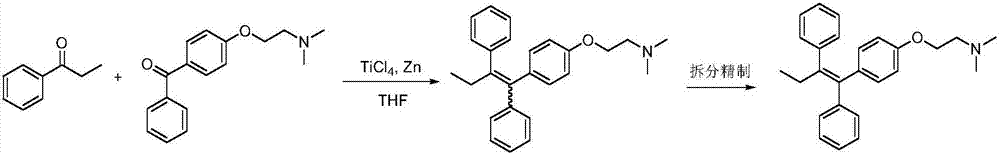
Preparation method of tamoxifen
A technology of tamoxifen and catalyst, which is applied in the field of preparation of tamoxifen, can solve the problems of low yield and long working hours, and achieve the effects of high yield, good selectivity and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
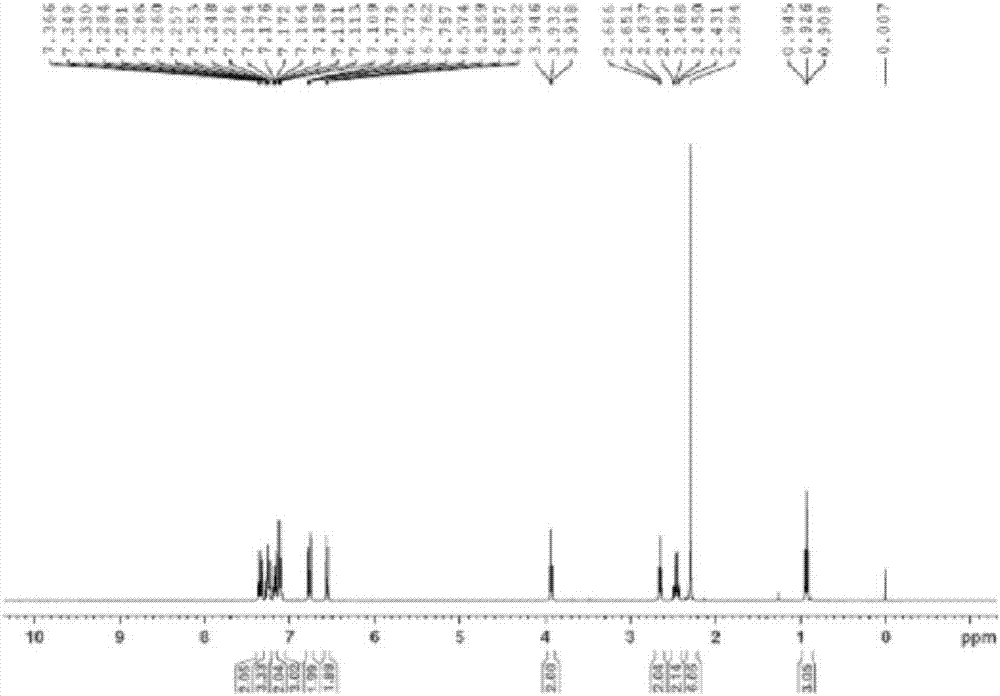
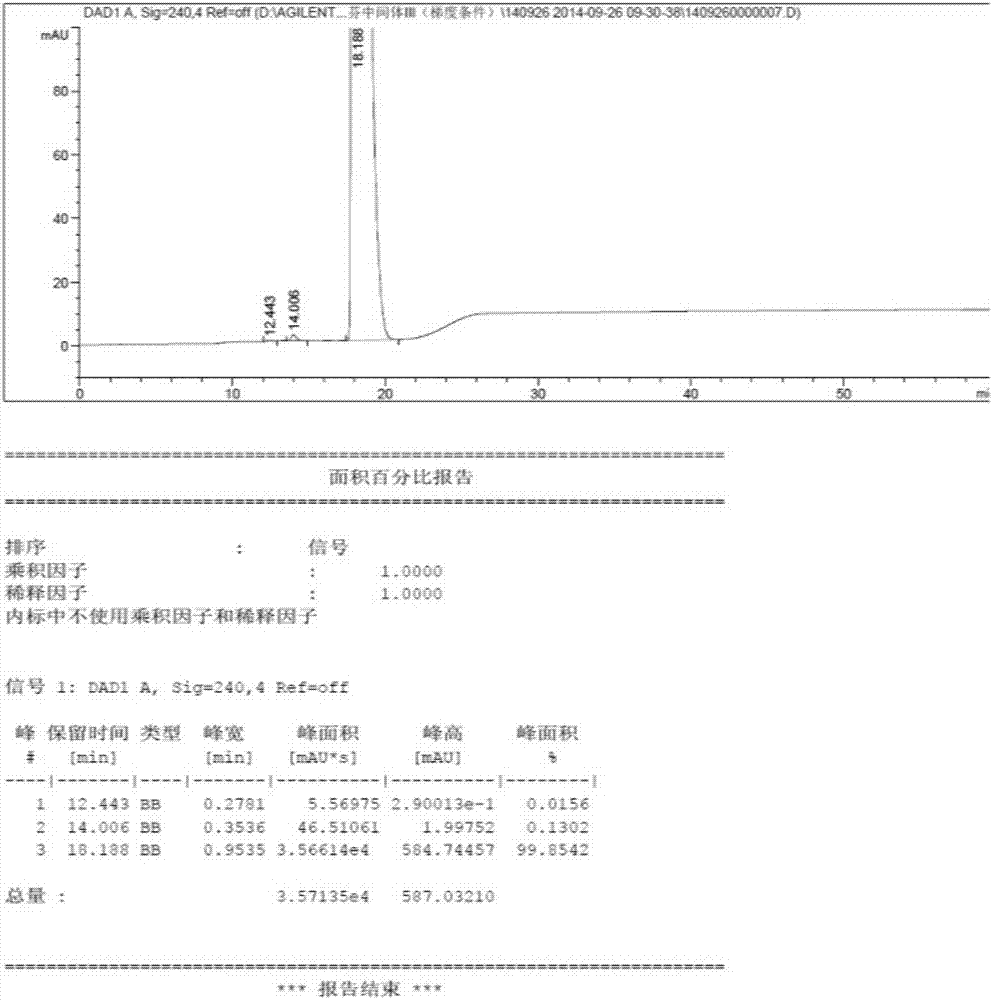
[0028] In a 1L hydrogenation reactor, add 40g toremifene, 400g methanol, 8.3g potassium hydroxide, 4g 10% palladium carbon, replace with 0.2MPa nitrogen for 3 times, then replace with 0.2MPa hydrogen for 3 times, keep the hydrogen The pressure is 0.3-0.5MPa, the reaction temperature is 70°C, and the reaction is stirred for 14 hours. Cool down to room temperature, filter with suction, distill off methanol under reduced pressure, add 250mL ethyl acetate and 100mL water to wash, stand to separate layers, wash the ethyl acetate layer with 100mL water again, stand to separate, and wash the organic phase with anhydrous sulfuric acid Dry over sodium, filter and distill off ethyl acetate under reduced pressure, add 120mL of acetone to heat and dissolve, then place it at 0°C for crystallization overnight, filter it with suction, and dry it with air at 60°C to obtain 31.5g of white needle-like solid tamoxifen, the yield 86%.Mp:96.5~98℃, 1 H NMR (400MHz, CDCl 3 ): δ0.93(t, J=7.6Hz, 3H,...
Embodiment 2
[0030] Into a 1L hydrogenation reactor, add 40g toremifene, 400g ethanol, 7.1g sodium hydroxide, 4g 10% platinum carbon, replace with 0.2MPa nitrogen for 3 times, then replace with 0.2MPa hydrogen for 3 times, keep the hydrogen The pressure is 0.3-0.5MPa, the reaction temperature is 90°C, and the reaction is stirred for 12 hours. Cool down to room temperature, filter with suction, distill ethanol off under reduced pressure, add 250mL ethyl acetate and 100mL water to wash, stand to separate layers, wash the ethyl acetate layer with 100mL water again, stand to separate, and wash the organic phase with anhydrous sulfuric acid Dry over sodium, filter and distill off ethyl acetate under reduced pressure, add 120mL of acetone to heat and dissolve, place it at 0°C for crystallization overnight, filter it with suction, and dry it with air at 60°C to obtain 31.1g of white needle-like solid tamoxifen, yield 84.9%. Mp: 96.3-97.8°C.
Embodiment 3
[0032] In a 1L hydrogenation reactor, add 40g toremifene, 400g ethanol, 8.3g potassium carbonate, 3g 10% palladium carbon, replace 3 times with 0.2MPa nitrogen gas, then replace 3 times with 0.2MPa hydrogen gas to keep the hydrogen pressure 0.5-0.7MPa, reaction temperature 90°C, stirring for 12 hours. Cool down to room temperature, filter with suction, distill ethanol off under reduced pressure, add 250mL ethyl acetate and 100mL water to wash, stand to separate layers, wash the ethyl acetate layer with 100mL water again, stand to separate, and wash the organic phase with anhydrous sulfuric acid Dry over sodium, filter and distill off ethyl acetate under reduced pressure, add 120mL of acetone to heat and dissolve, place it at 0°C for crystallization overnight, filter it with suction, and dry it with air at 60°C to obtain 32g of white needle-shaped solid tamoxifen, yield 87.5 %. Mp: 96-97.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com