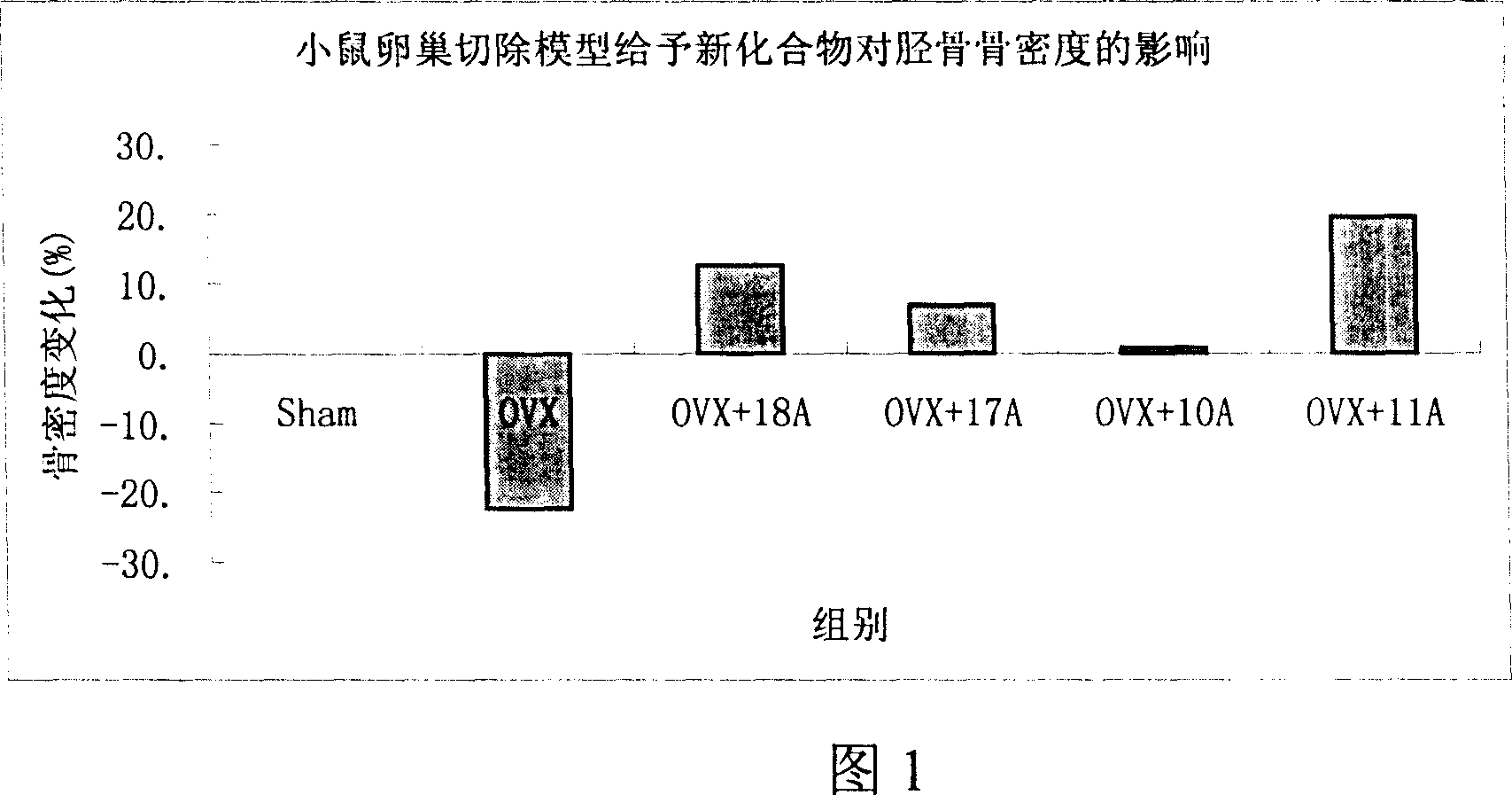
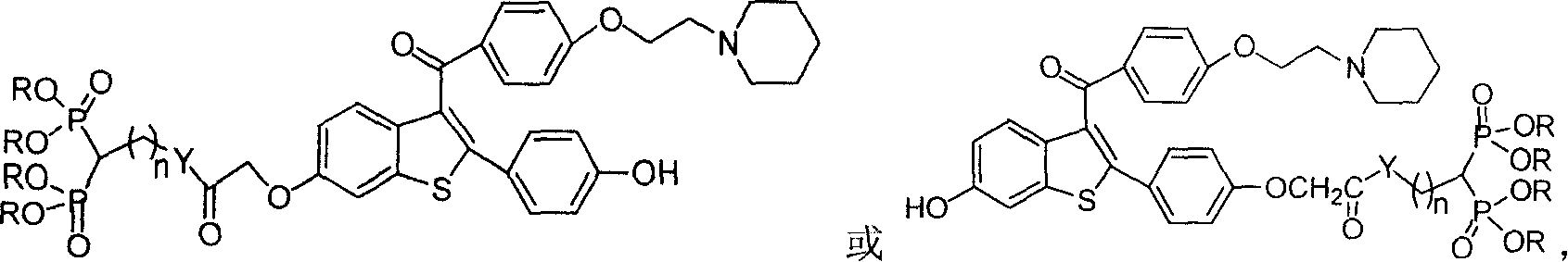
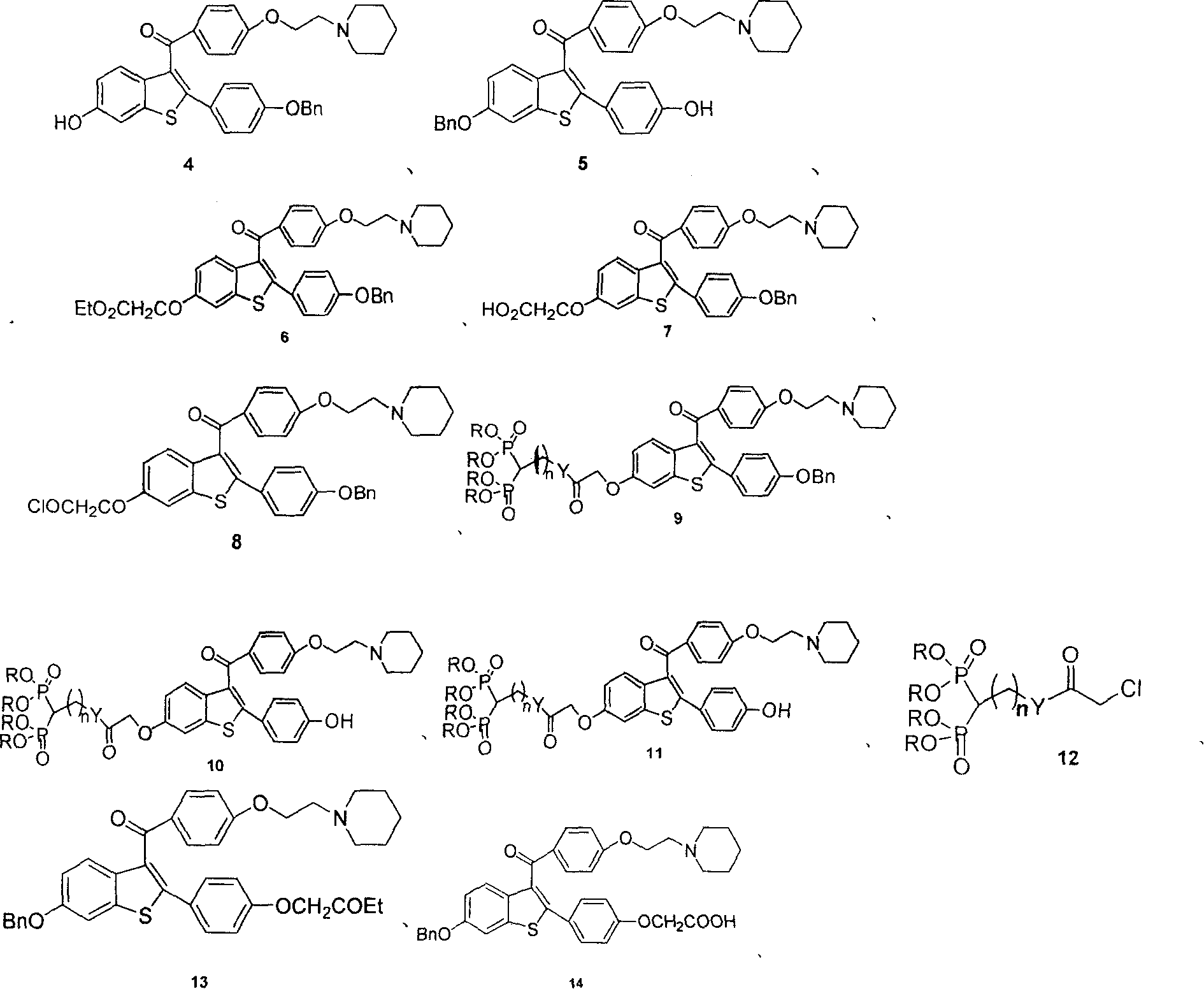Medication towards target of bone, synthetic method and application
A synthetic method and bone-targeted technology, applied in drug combinations, pharmaceutical formulations, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Raloxifene and bisphosphonic acid complex (9A) synthetic route 1
[0047] Step 1: Preparation of compound (6)
[0048] Method A:
[0049] 124mg (0.2mmol) of compound (4) was dissolved in 30ml of acetone, followed by adding 110mg of potassium carbonate, a small amount of potassium iodide and 345mg of ethyl chloroacetate, stirred at 60-70°C for 3 hours, concentrated, extracted three times with ethyl acetate, washed with water, anhydrous It was dried over sodium sulfate, concentrated by filtration, and subjected to silica gel column chromatography (chloroform / methanol 40:1) to obtain 65 mg of yellow powdery crystals with a yield of 52%.
[0050] Method B:
[0051] Dissolve 112.6 mg (0.2 mmol) of compound (4) in 4 mL of anhydrous THF, add 15 mg (0.35 mmol) of NaH, stir, then add 0.026 mL (0.23 mmol) of ethyl bromoacetate, and react for 1 hour. Concentrate, pour into saturated ammonium chloride solution, extract with appropriate amount of ethyl acetate, and dry over anhyd...
Embodiment 2
[0077] Example 2 Preparation of compound (11B). (preparation method is identical with embodiment 1)
[0078] Step 1: Preparation of compound (9B)
[0079] Using tetraethyl 4-aminobutyl-1,1-bisphosphonate as raw material, a light yellow solid was obtained with a yield of 80%.
[0080] 1 HNMR (400MHz, CDCl 3 )δ 1.3 (12H, br), 1.5 (6H, m), 1.7 (2H, qt, J=7), 1.9 (2H, tq, J=9.6), 2.4 (1H, tt, J=24.3) 2.53 (4H, br), 2.62 (2H, t), 2.79 (2H, t, J=5.6), 4.18 (10H, m), 4.65 (2H, s), 4.99 (2H, s), 6.77 (2H, d , J=9.0), 6.9 (2H, q, J=2.0, J=6.9), 7.00 (1H, dd, J=2.3, J=9.0), 7.08 (1H, d, J=2.0), 7.36 (7H , m), 7.6 (1H, d, J=9.0), 7.74 (2H, q, J=1.8, J=7.0).
[0081] Step 2: Preparation of compound (10B)
[0082] Yield 75%.
[0083] 1 HNMR (600MHz, CDCl 3 )δ1.35 (12H, m), 1.50 (6H, br), 1.71 (2H, m), 1.9 (2H, m), 2.38 (1H, m, J=23.6), 2.6 (2H, t), 2.64 (4H, m), 2.83 (2H, br), 4.2-4.61 (10H, m), 4.61 (2H, s), 6.6 (4H, t, J=9.1), 7.0 (1H, dd, J=2.4, J=8.8), 7.1 (2H, d...
Embodiment 3
[0087] Example 3 Preparation of compound (11C). (preparation method is identical with embodiment 1)
[0088] Step 1: Preparation of compound (9C)
[0089] Using tetraethyl 4-hydroxybutyl-1,1-bisphosphonate as raw material, a light yellow solid was obtained with a yield of 65%.
[0090] 1 HNMR (400MHz, CDCl 3 )δ1.23 (12H, br), 1.5 (6H, m), 1.8 (2H, qt, J=2), 2.1 (2H, tq, J=9), 2.4 (1H, tt, J=23.8) 2.53 (4H, br), 2.79 (2H, t, J=5.6), 3.62 (2H, t), 4.18 (10H, m), 4.65 (2H, s), 4.99 (2H, s), 6.77 (2H, d , J=9.0), 6.9 (2H, q, J=2.0, J=6.9), 7.00 (1H, dd, J=2.3, J=9.0), 7.08 (1H, d, J=2.0), 7.36 (7H , m), 7.6 (1H, d, J=9.0), 7.74 (2H, q, J=1.8, J=7.0).
[0091] Step 2: Preparation of compound (10C)
[0092] A light yellow solid was obtained with a yield of 78%.
[0093] 1 HNMR (600MHz, CDCl 3 )δ1.35 (12H, m), 1.50 (6H, br), 1.8 (2H, m), 2.1 (2H, m), 2.38 (1H, m, J=23.9), 2.64 (4H, m), 2.83 (2H, br), 3.7 (2H, t), 4.2-4.61 (10H, m), 4.61 (2H, s), 6.6 (4H, t, J=9....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com