Insulin sustained-release oral patch as well as preparation method and application thereof
A technology of insulin and pharmaceutical preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of patients' pain and complications, and achieve prolonged action time and half-life , highly feasible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
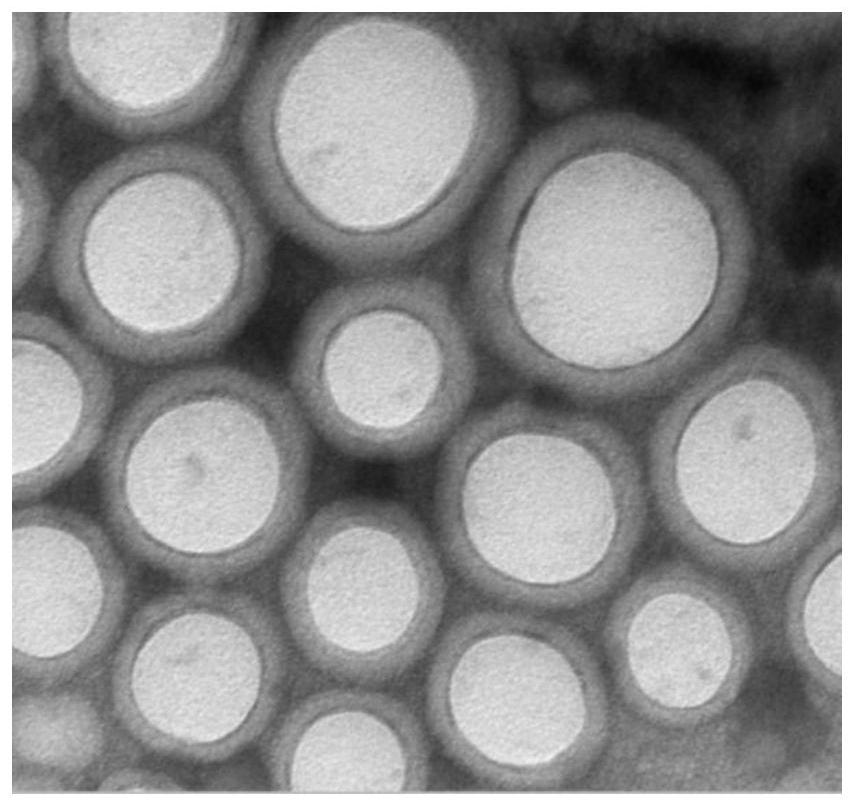
Image
Examples
Embodiment 1
[0062] Preparation of Insulin Sustained Release Oral Patch
[0063] (1) Polylactic acid-glycolic acid copolymerization (PLGA) solution preparation
[0064] Dissolve 120mg of polylactic acid-glycolic acid copolymer (50:50) in 2ml of dichloromethane to form an organic phase (0.06g / ml);
[0065] (2) Preparation of insulin solution
[0066] Accurately weigh 2 mg of solid insulin powder, dissolve in 190 μl of acetate buffer solution with pH=4, and fully dissolve into a transparent liquid.
[0067] (3) Preparation of insulin dispersion solution
[0068] 190 μl of insulin liquid was evenly dispersed in 250 μl of sodium alginate solution with a mass volume ratio of 0.003% (w / v) to form an internal aqueous phase.
[0069] (4) Preparation of colostrum
[0070] Inject the internal aqueous phase into the organic phase, and perform ultrasonic emulsification for 1 min under ultrasonic conditions of 10-1000w to form colostrum (W / O); the volume ratio of the organic phase to the internal a...
Embodiment 2
[0085] Insulin Nanoparticle Stability Test
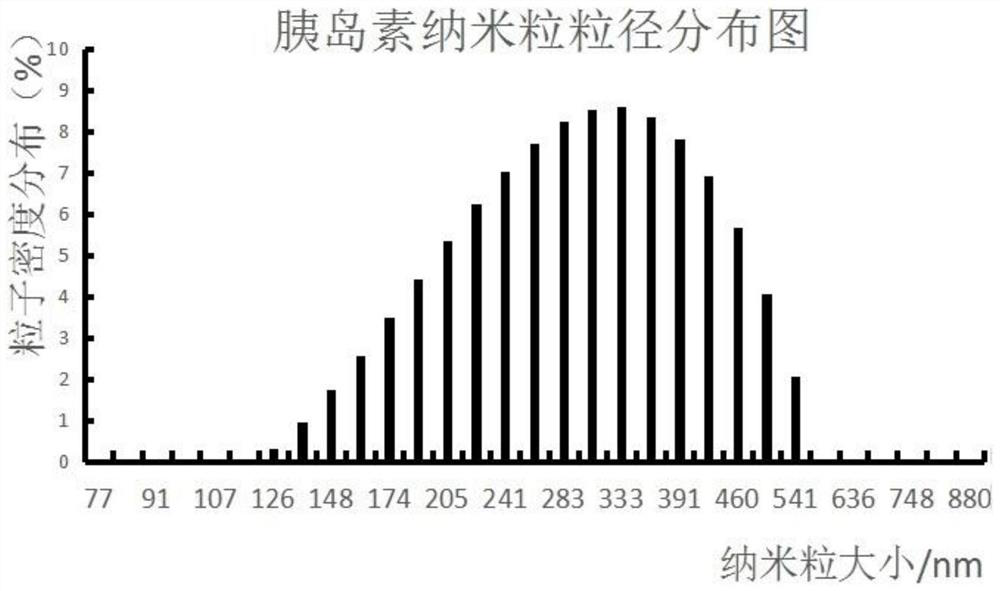
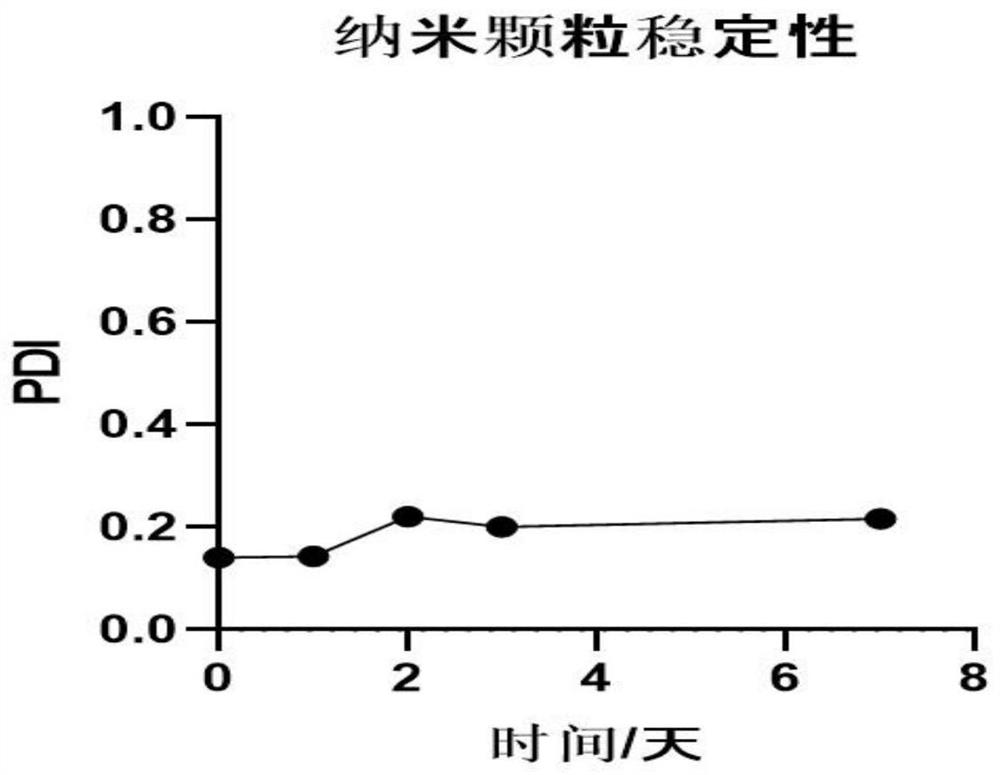
[0086] The prepared insulin nanoparticles were subjected to PDI (particle distribution density) detection at the same time on days 1-7. The storage condition of nanoparticles is 4°C, and it is observed whether aggregation or degradation occurs. After detection by Anton Paar LITESIZER 500 particle size analyzer according to the principle of dynamic light scattering (DLS), the PDI value is 0.1-0.3, indicating good dispersion.
[0087] image 3 It is the stability change of the insulin nanoparticle of the present invention; it can be drawn from the figure that the insulin nanoparticle has good stability when stored at 4° C., and no obvious aggregation and degradation phenomenon occurs.
Embodiment 3
[0089] Detection of hypoglycemic activity of insulin lozenges
[0090] The diabetic rat model was induced by intraperitoneal injection of STZ. The established rat models were randomly divided into six groups A, B, C, D, E, and F, with 6 rats in each group, and were fed normally for one week, and fasted for 12 hours before administration.
[0091] Group A blank excipient group (sublingual / buccal patch), group B blank nanoparticle patch group (sublingual / buccal patch), group C insulin nanoparticle patch group (sublingual), group D insulin nanoparticle patch group (buccal patch), E group blank control group, F group positive control group (insulin injection group), after administration, at 0, 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 300, 360 , 420, and 480 min to take blood from the tail vein, and measure blood sugar with a blood glucose meter and blood glucose test strips. During the experiment, all groups were anesthetized and anesthesia was supplemented appropriately dur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com