Long-acting single-chain insulin analog and conjugate thereof
A technology for single-chain insulin and analogs, which can be used in insulin, hormone peptides, animal/human proteins, etc., and can solve the problems of increased production process, complexity, and unsuitability for the preparation of single-chain insulin analogs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0270] Embodiment 1: Preparation of single-chain insulin analog expression vector
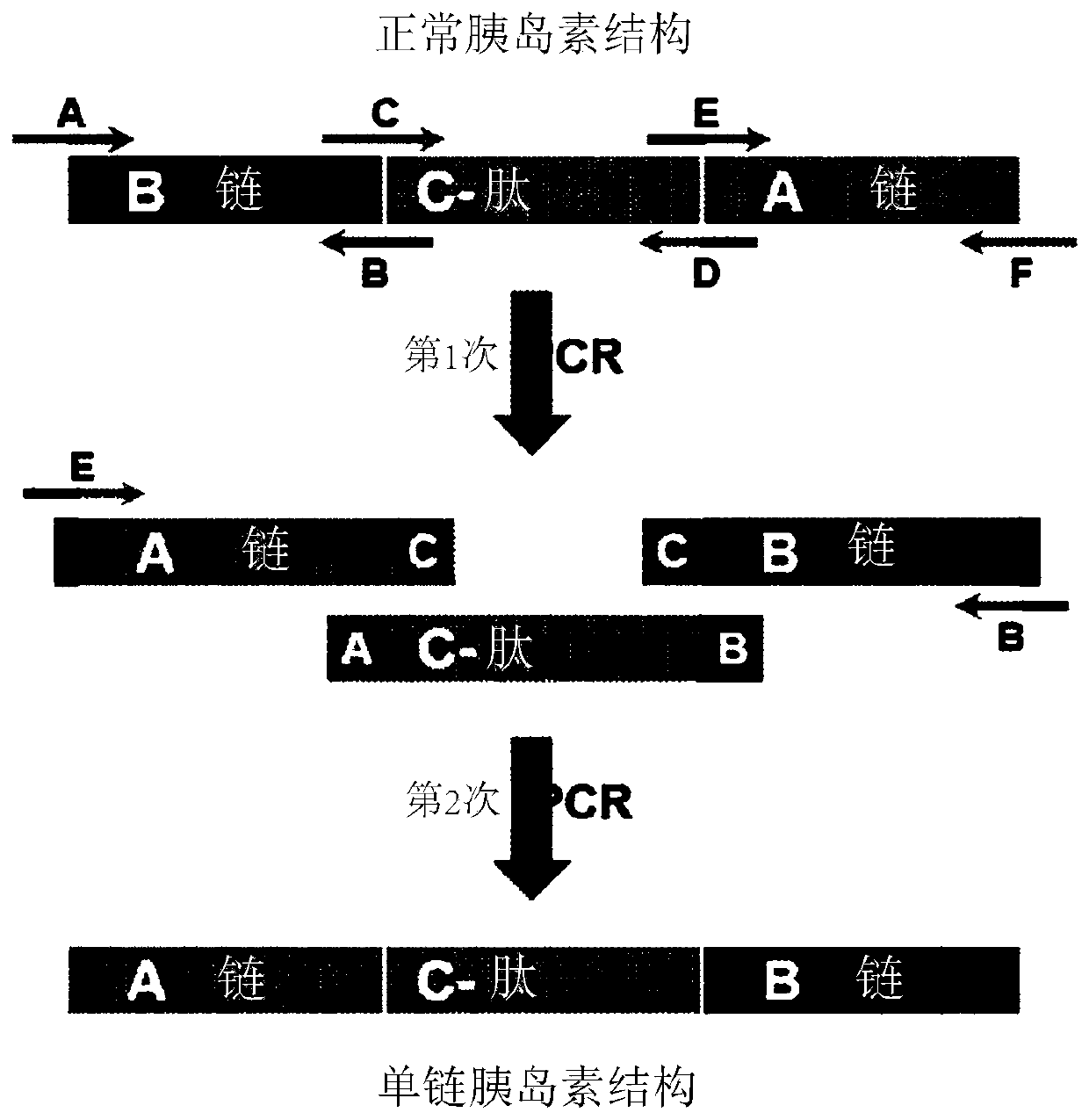
[0271] 1. Preparation of Proinsulin Analogs (A Chain-C Peptide-B Chain)
[0272] Expression vectors with nucleotide sequences were prepared to allow expression of insulin analogs and proinsulin analogs in E. coli. In order to release (free) the N-terminus of the insulin A chain, the expression vector includes a protein capable of expressing native proinsulin (B chain-C peptide-A chain; SEQ ID NO:4 and 5) and other proinsulin analogs (A chain-C Peptide-B chain, nucleotide sequence of SEQ ID NO: 6 and 7), and was prepared as follows.
[0273] [Table 1]
[0274]
[0275]
[0276] In order to prepare an expression vector encoding 86 amino acid proinsulin analogs, six primers were synthesized based on the reported proinsulin gene sequence (NM_000207.2, NCBI). In addition, using proinsulin cDNA (Origene) as a template, chain A, peptide C and chain B were respectively amplified by PCR, an...
Embodiment 2
[0308] Example 2: Expression of single chain insulin analogs
[0309] Under the control of the T7 promoter, the expression vector constructed in the above-mentioned Example 1 was used to express the recombinant single-chain insulin analogue. Escherichia coli BL21DE3 (E. coli B F-dcm ompT hsdS(rB-mB-)galλ(DE3); Novagen) was transformed with each recombinant single-chain insulin analog expression vector. For the transformation method, the method recommended by Novagen was used. Each transformed single colony transformed with each recombinant expression vector was collected, inoculated into 2X Luria Broth medium containing ampicillin (50 μg / mL), and incubated at 37° C. for 15 hours. Recombinant strain cultures were mixed with 2X LB medium containing 30% glycerol at a ratio of 1:1 (v / v), and 1 mL each was dispensed into cryo-tubes and stored at -140°C. The resultant was used as cell stock for production of recombinant fusion protein.
[0310]For expression of recombinant sing...
Embodiment 3
[0311] Example 3: Extraction and renaturation of recombinant single-chain insulin analogs
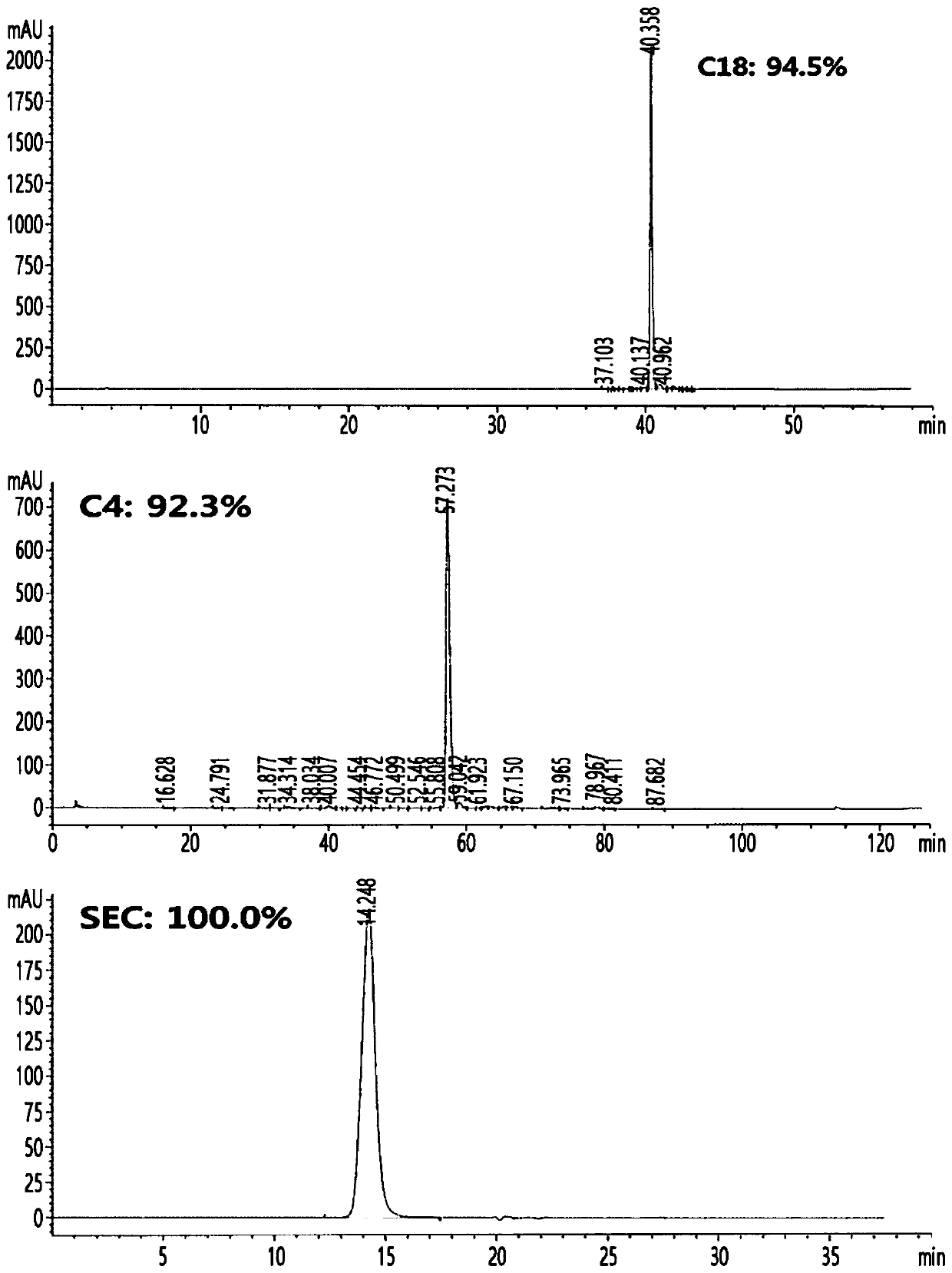
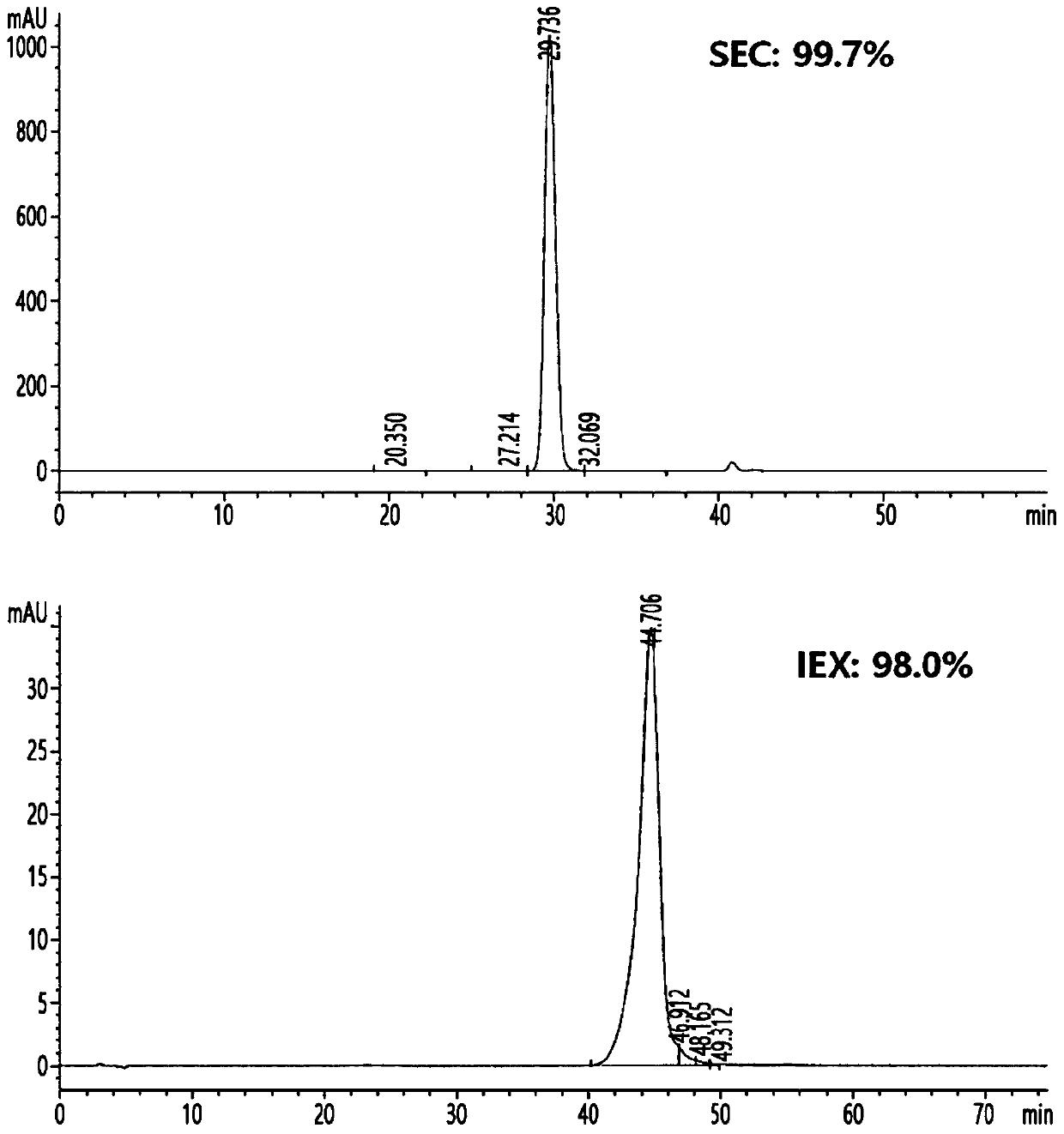
[0312] Cells from E. coli expressing the single-chain insulin analog obtained in the above examples were disrupted and renatured to convert the single-chain insulin analog into a soluble form. Cell pellets equivalent to 1L of culture were suspended in 1L of lysis buffer (20mM Tris-HCl pH 9.0, 1mM EDTA pH 8.0, 0.2M NaCl, 0.5% Triton X-100) ) machine disrupted the recombinant E. coli at 15,000 psi. After centrifugation at 12,000 g for 30 minutes, the supernatant was discarded, and the pellet was washed with 1 L of lysis buffer (50 mM Tris-HCl pH 9.0, 1 mM EDTA pH 9.0, 0.2M NaCl, 0.5% Triton X-100). After centrifugation under the same conditions as above, the supernatant was discarded, and the pellet was resuspended with distilled water. After centrifugation under the same conditions, washed E. coli inclusion body pellets were obtained. The washed inclusion body pellet was resuspended...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com