A kind of antitumor composition inhalation powder spray and preparation method thereof
A technology for inhaling powder mist and composition, which is applied in the field of pharmaceutical preparations, and can solve problems such as low bioavailability, insufficient anticancer efficacy, and large toxic and side effects of VCR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The experimental materials and methods used in the examples are as follows:
[0041] Experimental cells: human T lymphocytic leukemia cells (Jurkat), human breast cancer cells (MCF-7), retinoblastoma cell line (HXO-Rb44).
[0042] Growth medium: RPMI-1640 medium (containing 10% FBS+1% PS).
[0043] Culture environment: 37°C, 5% CO 2 Saturated humidity incubator.
[0044] Human T lymphocytic leukemia cells (Jurkat), human breast cancer cells (MCF-7), and retinoblastoma cell lines (HXO-Rb44) in logarithmic growth phase and in good shape were washed once with PBS buffer and added Digest with 0.25% trypsin solution and gently pipet to a single cell suspension. Pipette 20 μl of the cell suspension to a cell counting plate for cell counting. The above cell suspension was 1×10 cells 4 The density of cells / well was inoculated into 96-well plates, with 5 duplicate wells in each group, at 37°C, 5% CO. 2 The cells were cultured in a saturated humidity incubator for 24 h to m...
Embodiment 2
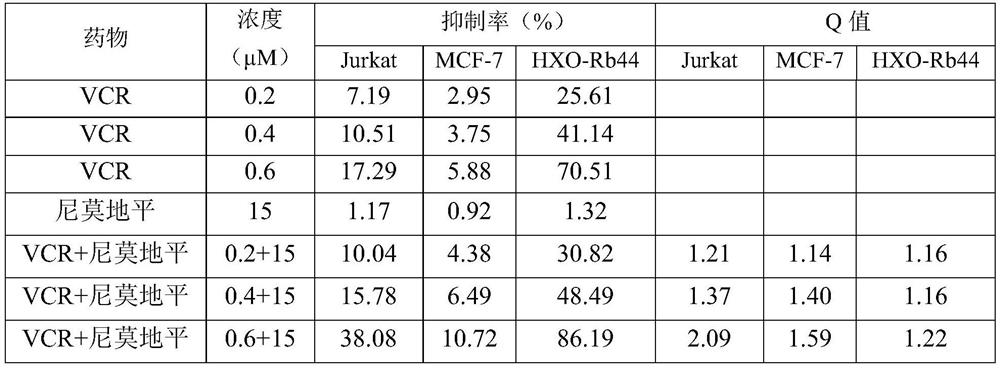
[0054] NOD-SCID mice were randomly divided into control group (with normal saline), VCR group, nimodipine group and VCR-nimodipine combination group, 10 mice in each group. 7.5Gy dose given to mice 60 After 5 days of whole-body irradiation with Coγ rays, human T lymphocytic leukemia cells (Jurkat) in logarithmic growth phase and in good shape were taken, washed three times with PBS buffer, digested with 0.25% trypsin solution, and gently pipetted to make them Drop off, add 12ml RPMI-1640 medium, transfer the suspension to a 15ml centrifuge tube, and centrifuge at 1000rpm for 5min. The supernatant was discarded, and the pelleted cells were washed with sterile saline and diluted. Take 40 μl of cell suspension for microscopic examination and count, and make a concentration of 1×10 6 cells / ml of tumor cell suspension, 0.2ml / cell was injected into the tail vein. The mice were observed for leukemia symptoms after inoculation with Jurkat cells, and the drug was administered on the...
Embodiment 3
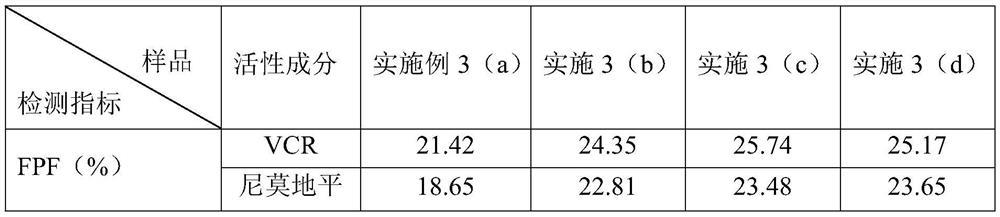
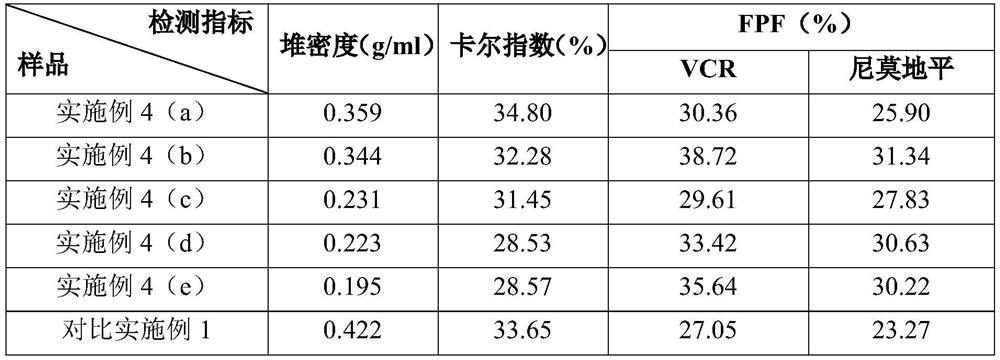
[0060] (a) Take 3.0g of VCR and 25g of nimodipine and carry out ultrafine jet pulverization respectively, set the injection pressure of 7bar, the pulverization pressure of 5bar, and the feed rate of 0.4g / min to obtain micronized VCR (D10=1.29 μm, D50=3.62 μm, D90=6.49 μm, NLT96.44%<10 μm) and nimodipine (D10=1.48 μm, D50=3.75 μm, D90=6.76 μm, NLT96.12%<10 μm). Mix VCR 0.8g (micropowder), nimodipine 12g (micropowder), mannitol 50g and leucine 0.4g, place the mixed sample in a high-speed mixing granulator, set the stirring speed to 1300rpm, and finish after mixing for 20min. After settling for 1 h, the resulting dry powder mixture was filled into No. 3 dark vegetable capsules in aliquots.
[0061] (b) get 3.0g VCR and 0.03g leucine and mix, get 25g nimodipine and 0.25g leucine and mix, the above-mentioned two mixtures are respectively carried out ultrafine jet pulverization according to the parameter of embodiment 3 step (a), A mixture of micronized VCR (D10=1.13 μm, D50=3.05 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com