Frovatriptan inhalation aerosol powder, preparation method thereof and application of powder
A technology for inhaling powder spray and frovatriptan, which is applied in the field of pharmaceutical preparations, can solve the problems of low bioavailability and slow onset of action, achieve good lung deposition rate, facilitate storage, and avoid the first-pass effect of the liver Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
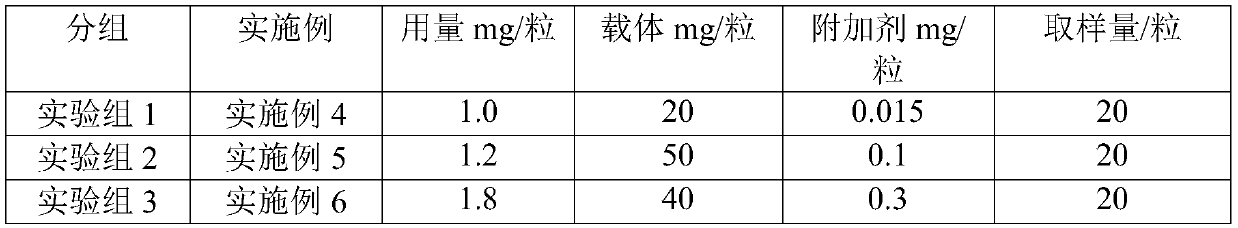
Embodiment 1
[0031] 1.0 g of frovatriptan micropowder and 20 g of lactose obtained after fluidized bed granulation were uniformly mixed in equal increments and then packed in No. 3 capsules.
Embodiment 2
[0033] 1.2 g of frovatriptan micropowder and 50 g of lactose obtained after fluidized bed granulation were uniformly mixed in equal increments and then packed in No. 3 capsules.
Embodiment 3
[0035] 1.8 g of frovatriptan micropowder and 40 g of lactose obtained after fluidized bed granulation were uniformly mixed in equal increments and then packed in No. 3 capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com