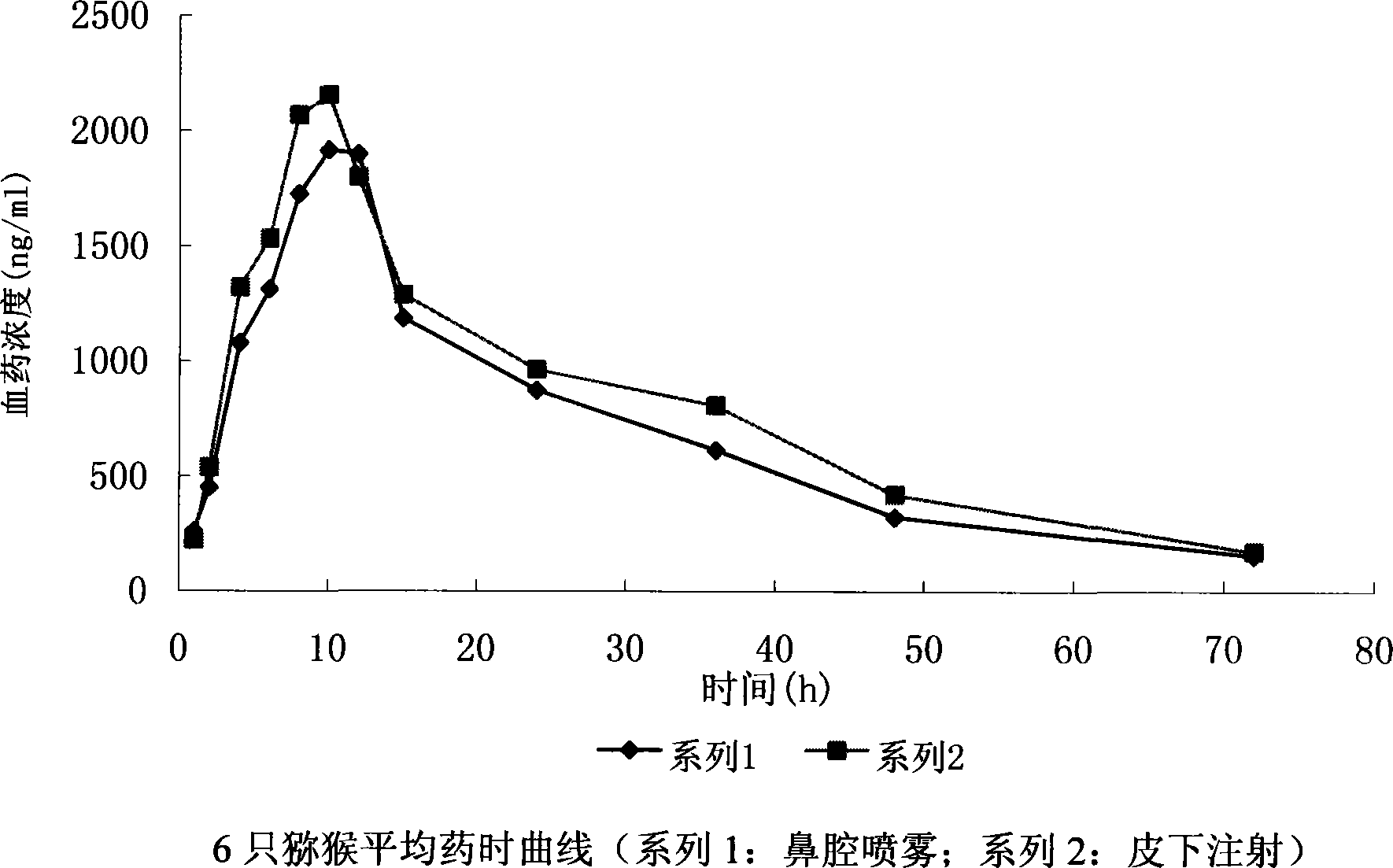
Reeombinnt human granulocyte colony stimulating factor microemulsion
A technology of colony-stimulating factor and granulocytes, which is applied in the field of medicine, can solve problems such as the inability to effectively solve the administration method of recombinant human granulocyte-colony-stimulating factor, difficulties in the industrialization of the pharmaceutical industry, and complicated preparation processes, so as to avoid the first-pass effect, Extensive social and economic benefits, effects of avoiding inconvenience and pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] Seven kinds of specific prescriptions are as follows: (determine the auxiliary material type and quantity of microemulsion preparation)
[0023] Prescription A:
[0024] Recombinant Human Granulocyte Colony Stimulating Factor 1.0g
[0025] Absolute ethanol 1.0g
[0026] Propylene glycol 0.5g
[0027] Polyoxyethylene hydrogenated castor oil 4.0g
[0028] Glyceryl Linoleate 2.5g
[0029] water 1000ml
[0030] Make 1000ml microemulsion
[0031] Preparation process: Weigh 1.0g of rhG-CSF, add it to 2.5g of linoleic acid glyceride, stir to dissolve, then add 4.0g of polyoxyethylene hydrogenated castor oil, 1.0g of absolute ethanol, 0.5g of propylene glycol, and stir well , After dispersing in 1000ml water, the microemulsion is obtained.
[0032] Prescription B:
[0033] Recombinant Human Granulocyte Colony Stimulating Factor 1.0g
[0034] Absolute ethanol 1.0g
[0035] Macrogol 200 0.5g
[0036] Sorbitan Fatty Acid Ester 2.0g
[0037] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com