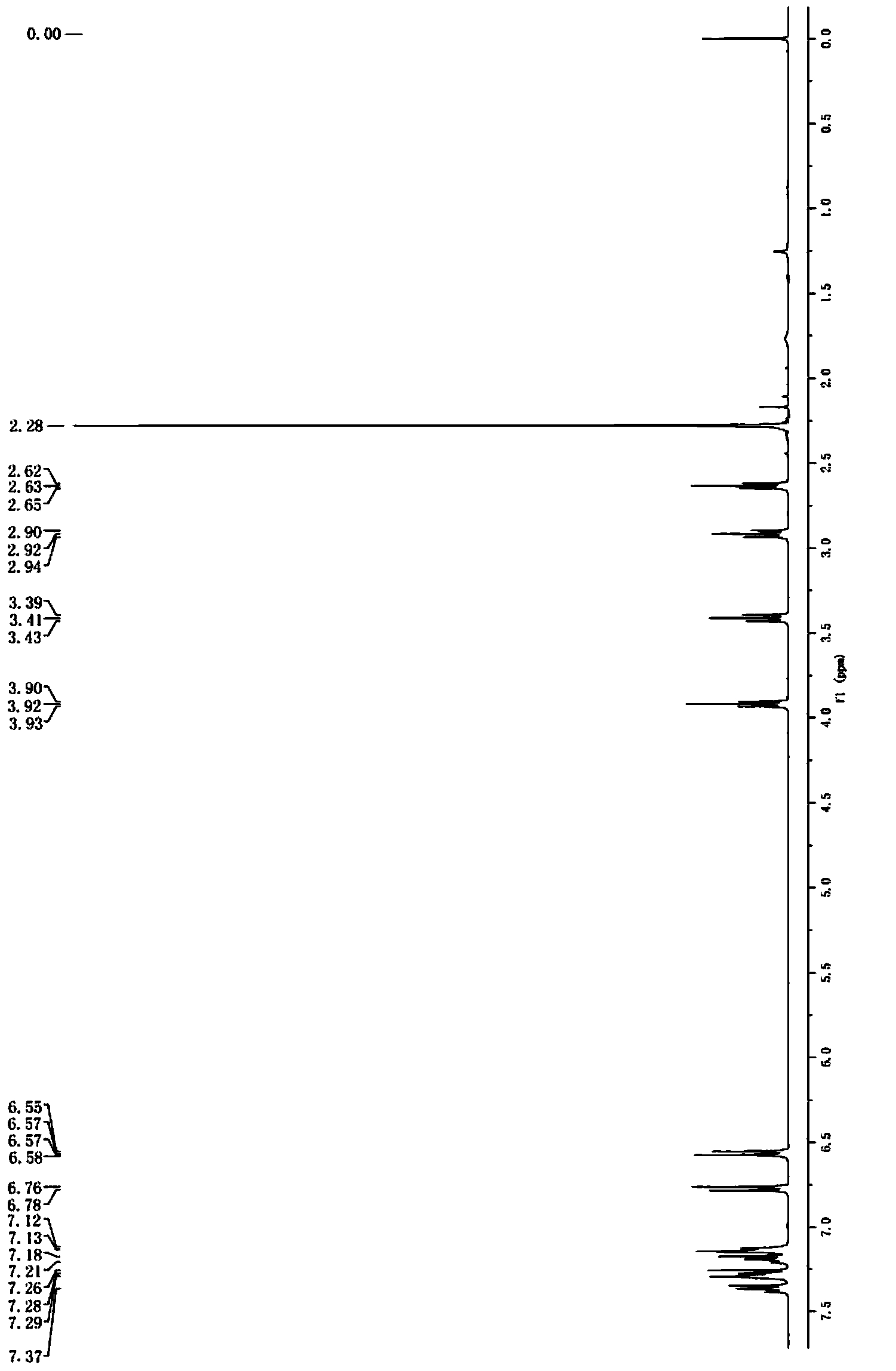Synthesis method of toremifene
A synthetic method, the technology of toremifene, applied in chemical instruments and methods, the preparation of organic compounds, the preparation of amino hydroxyl compounds, etc., can solve the problems of poor stereoselectivity and low product synthesis rate, and achieve easy separation and stereoselective High performance and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
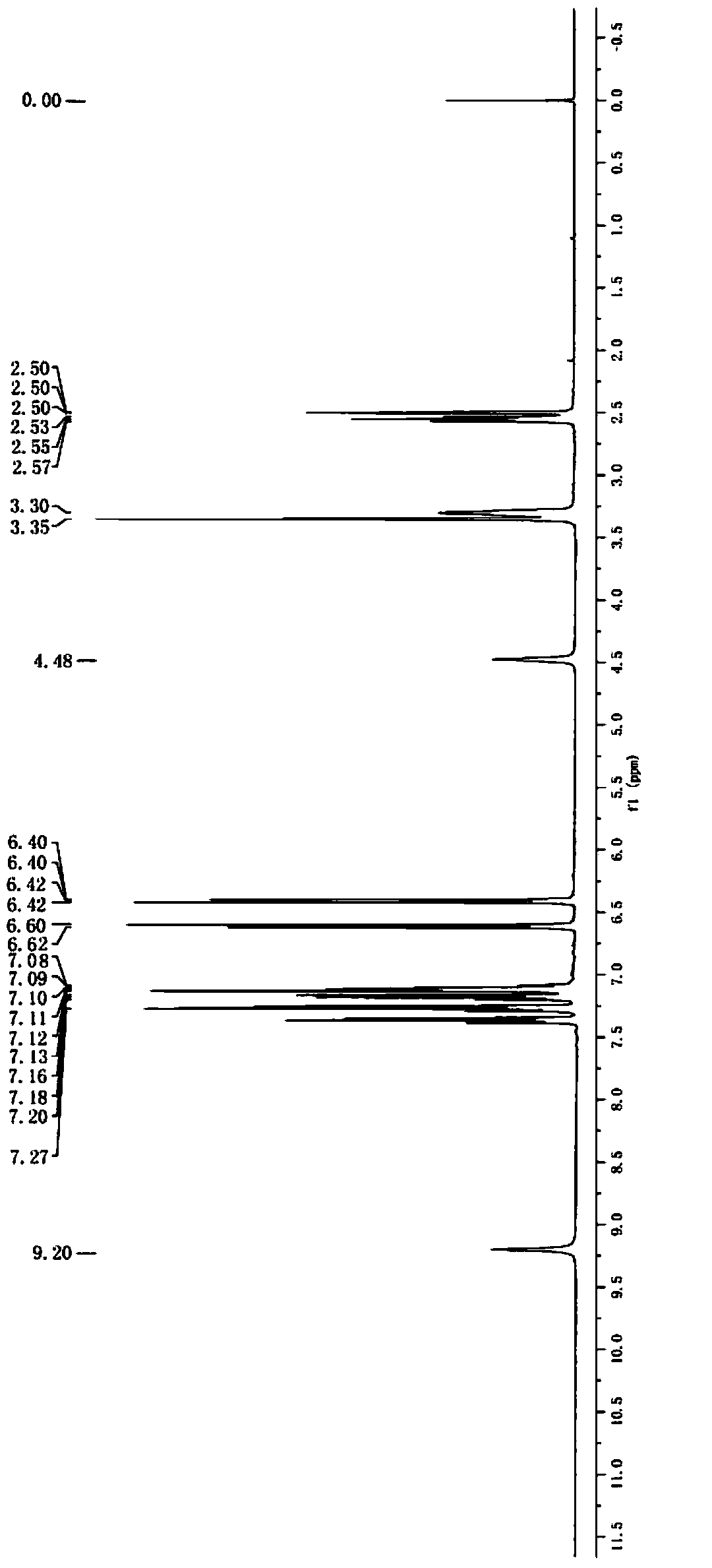
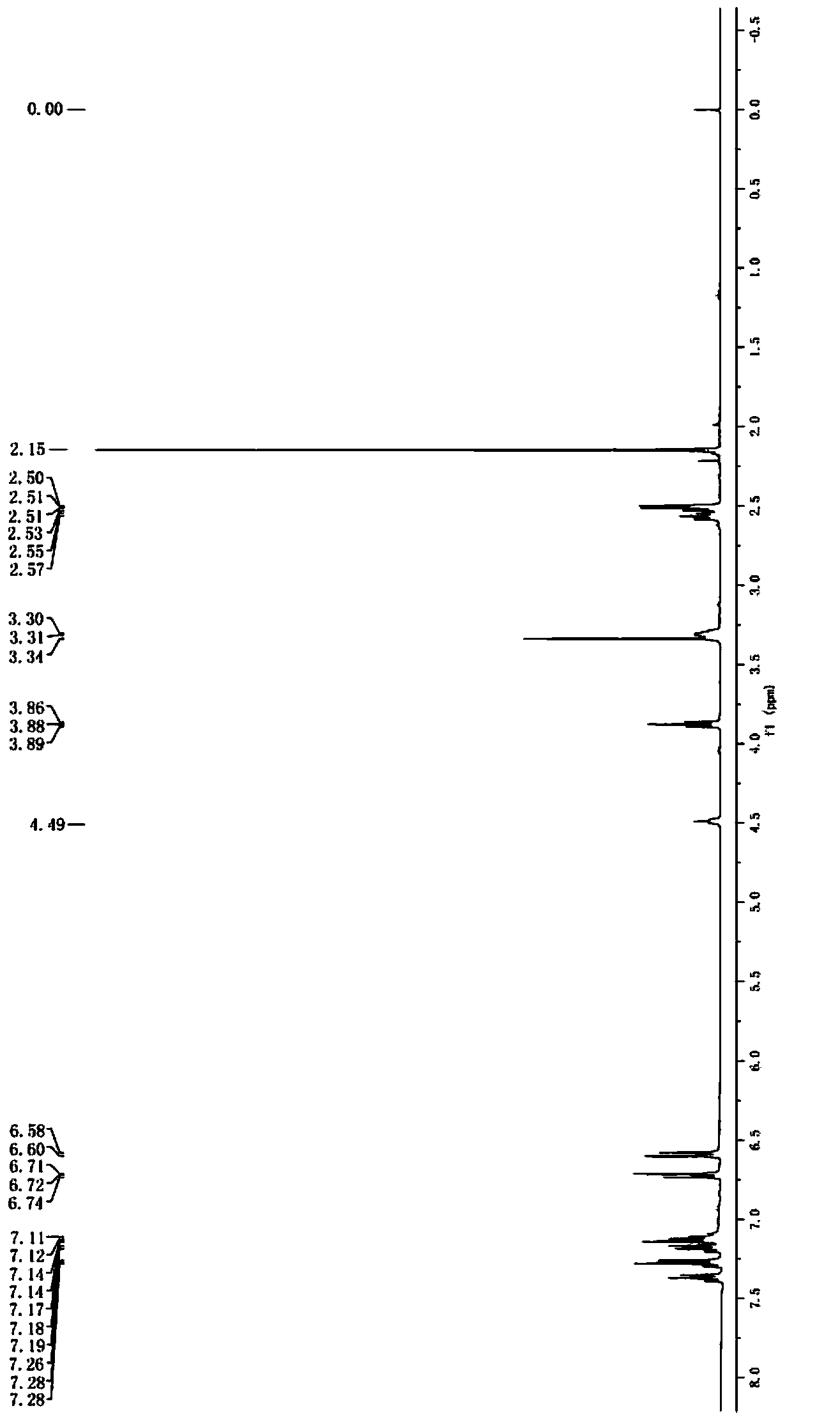
Image
Examples
preparation example Construction
[0033]In a typical embodiment of the present invention, a synthetic method of toremifene is provided, the synthetic method comprises: Step S1, making the compound B having the structural formula II react with the compound C having the structural formula III in a McMurray reaction , to obtain a compound D having the structural formula IV; Step S2, making the compound D and the compound E having the structural formula V or the hydrochloride of the compound E undergo a selective alkylation reaction on the phenolic hydroxyl group to obtain a compound F having the structural formula VI; Step S3, reacting compound F with thionyl chloride to obtain toremifene, wherein the structural formula II is: Structural formula III is: Structural formula IV is Structural formula Ⅴ is ClCH 2 CH 2 N(CH 3 ) 2 ; Structural formula Ⅵ is
[0034] The synthetic route of above-mentioned synthetic method is as follows:
[0035]
[0036] The above-mentioned synthetic route that the present ...
Embodiment 1
[0050] Synthesis of Z configuration compound D:
[0051] Add 130.8g (2mol) zinc powder and 1.5L THF to the 5L reaction flask, stir evenly to form the first mixed system, control the temperature of the first mixed system to be lower than -15°C, and slowly add 189.7g ( 1mol) of titanium tetrachloride, the dropwise addition is completed, and the second mixed system is formed after continuing to stir for 2 hours; the temperature of the second mixed system is controlled at 20-25°C, and 156.2g (0.8mol) Compound B and 1.5L tetrahydrofuran solution of 150g (1mol) compound C form a third mixed system. After the dropwise addition, the temperature of the third mixed system is raised to 60-65°C. After stirring for 5 hours, the temperature is lowered to 25°C to obtain the first Product system; slowly pour the first product system into 2L of water, add sodium carbonate to neutralize until the pH value of the first product system is between 9 and 10 to obtain the first neutralization system,...
Embodiment 2
[0057] Synthesis of Z configuration compound D:
[0058] Add 294.2g (4.5mol) of zinc powder and 2.25L of ethylene glycol dimethyl ether into a 5L reaction bottle, stir evenly to form the first mixed system, control the temperature of the first mixed system to be lower than -15°C, and slowly transfer to the first mixed system Add 462.6g (3mol) titanium trichloride dropwise to the mixture, after the dropwise addition is complete, continue to stir for 2 hours to form the second mixed system; control the temperature of the second mixed system to be 20-25°C, and slowly add dropwise the second mixed system containing 237.8g (1.2mol) of compound B and 150g (1mol) of compound C in 2.25L ethylene glycol dimethyl ether solution form a third mixed system, after the dropwise addition is completed, the temperature of the third mixed system is raised to 60-65°C, kept stirring for 5 Hours later, cool down to 25°C to obtain the first product system; slowly pour the first product system into 2L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com