Methods for the treatment of erectile dysfunction using fispemifene
a technology of erectile dysfunction and fispemifene, which is applied in the field of treatment of erectile dysfunction using fispemifene, can solve problems such as insufficient responsiveness, and achieve the effect of improving erectile function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
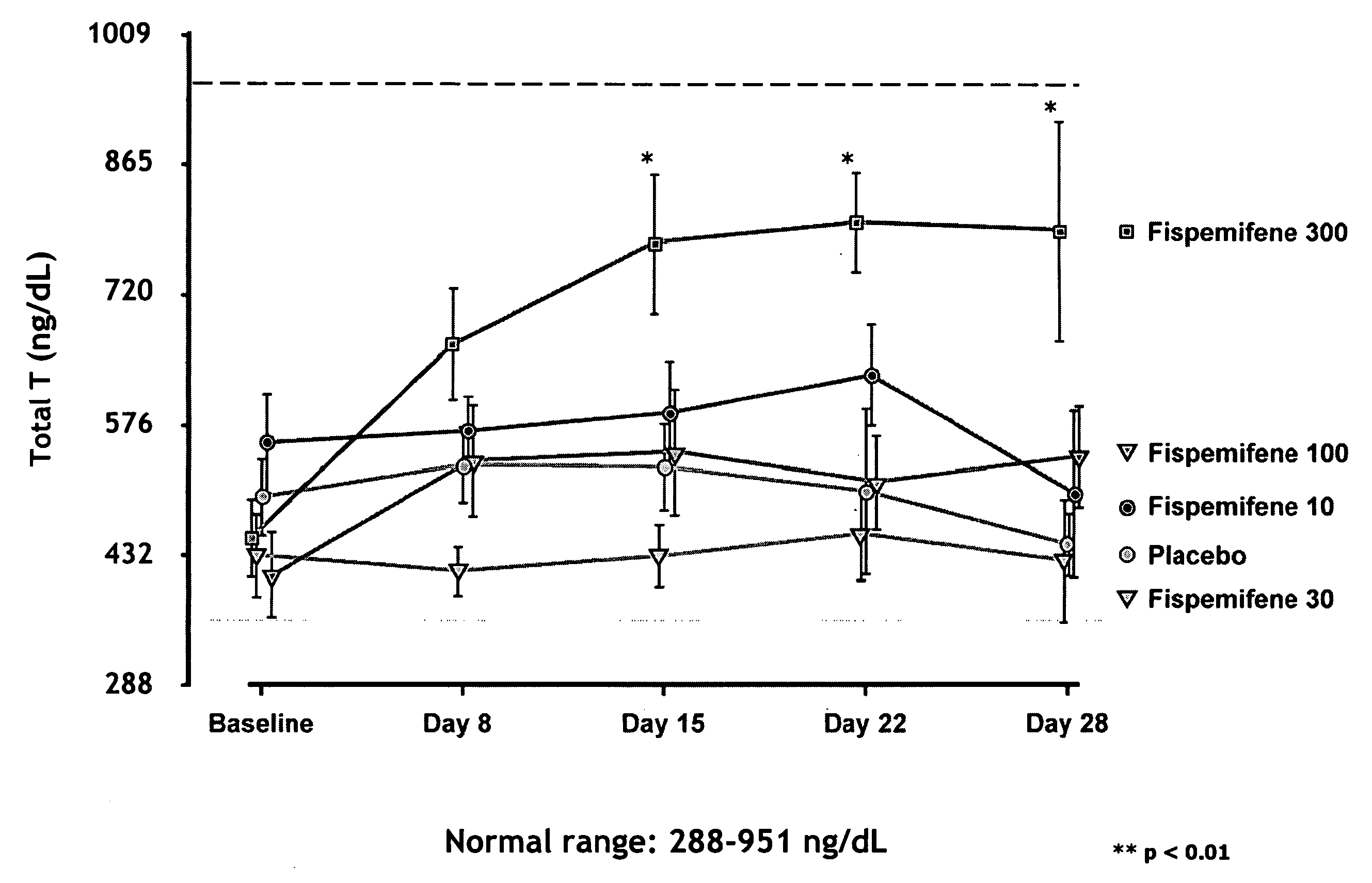
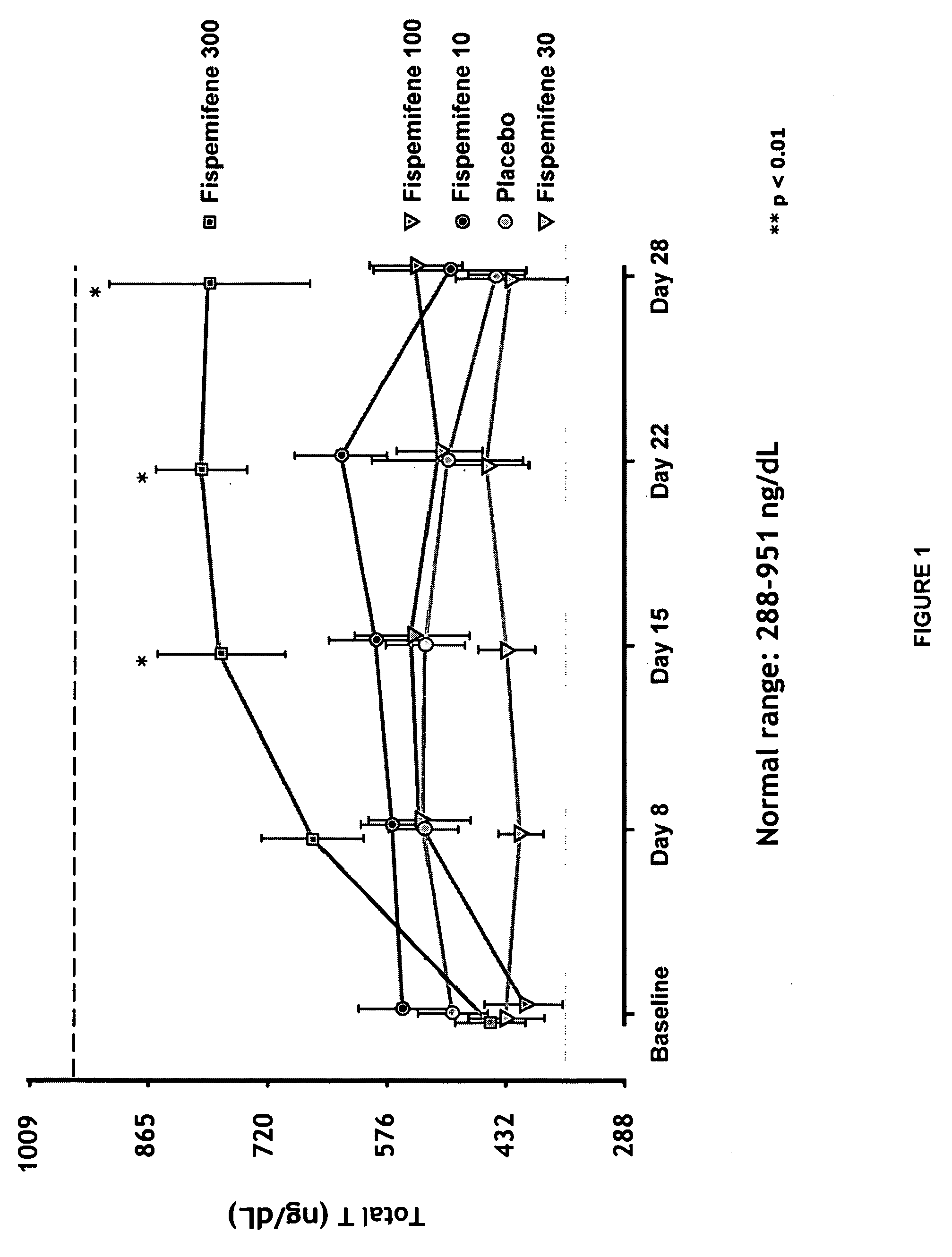
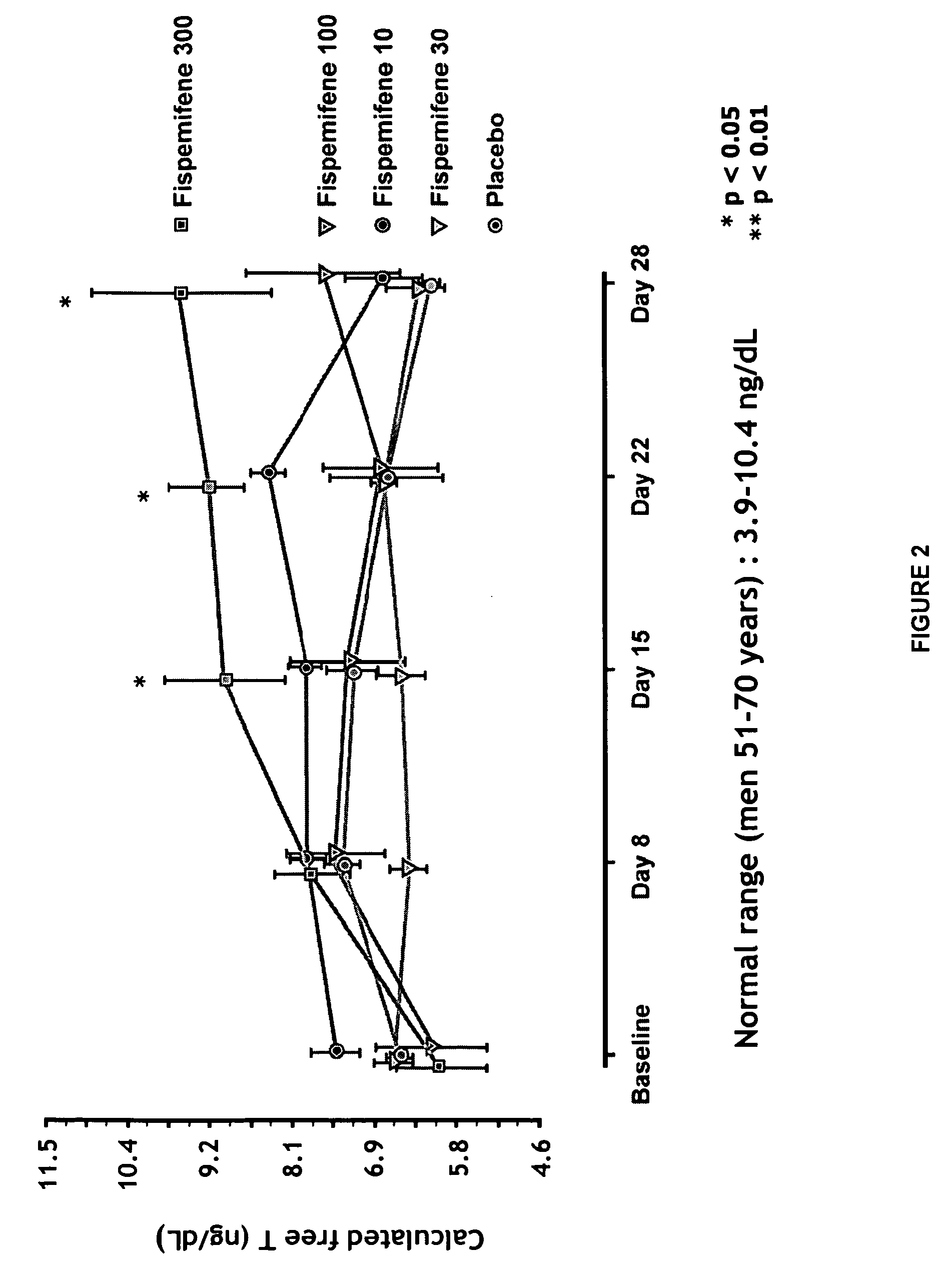
[0053]Fispemifene has been studied in two phase I studies in humans—in a single dose and a repeated dose study. Effect of fispemifene on hormone levels was one main focus of the repeated dose study. The phase I repeated dose study (number 101-50202) was a randomized, double-blind, placebo-controlled 28-day dose-escalation study performed in 31 healthy, elderly men, aged 50-68 years. The main objective of the study was to investigate the tolerability, safety and pharmacokinetics of fispemifene after repeated oral doses, but the study focused also on the effects of fispemifene on serum free and total testosterone, estradiol, and other relevant hormones. The fispemifene doses 10, 30, 100 and 300 mg per day and placebo were administered once every morning as capsules containing 10 mg or 100 mg of fispemifene, or placebo. The dose was escalated to the next higher dose level, if the previous dose had been safe and well tolerated evaluated by the laboratory safety determinations and ultras...
example 2
[0059]This is a prophetic example. Subjects would be selected from men, all over twenty years of age, unresponsive to PDE-5 inhibitor treatment as demonstrated by responses on the IIEF for a 28 day lead-in period, having morning total testosterone level less than or equal to 400 ng / dL. Half of the subjects would be assigned to the fispemifene treatment group (fispemifene plus sildenafil) and half will be assigned to the placebo control group (sildenafil without fispemifene). Subjects would self administer fispemifene once daily in the morning after breakfast for 8 weeks. Subjects would take sildenafil 100 mg on an as needed basis when sexual activity is anticipated.
[0060]The following observations would be expected: increases in total testosterone levels from baseline to week 4 to week 8; improvement in IIEF erectile function domain score from baseline to week 4 to week 8; improvement in other IIEF domain scores from baseline to week 4 to week 8.
example 3
[0061]A randomized, double-blind, placebo controlled, parallel-group study of once-daily doses of fispemifene (100, 200, and 300 mg / day) given for 4 weeks was conducted in a population of hypogonadal men. Subjects were required to meet all of the following inclusion criteria at screening and prior to randomization to be eligible for the study:
[0062]1. The subject had signed a written informed consent to participate in the study and had agreed to follow dosing instructions and complete all required study visits;
[0063]2. The subject was a male ≧40 years of age at the time of randomization.
[0064]3. The subject had a screening total testosterone level and a confirmatory baseline total testosterone level ≦350 ng / dL. Testosterone levels were determined from early morning (0700 h to 0900 h) specimens; and
[0065]4. The subject had a serum LH level of 1.7-15.0 IU / L and an FSH level of 1.5-15.0 IU / L at the screening visit.
[0066]Subjects were excluded from the study if they had an elevated seru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time points | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| equilibrium dialysis | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com