Preparation method of citric acid toremifene intermediates
A technology of toremifene citrate and its intermediates, which is applied in the field of medicine and chemical industry, and can solve the problem of inability to prepare toremifene citrate intermediates, non-conservation of valence states, and inability to prepare toremifene citrate Intermediates and other problems, to avoid self-condensation reaction, short reaction time, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
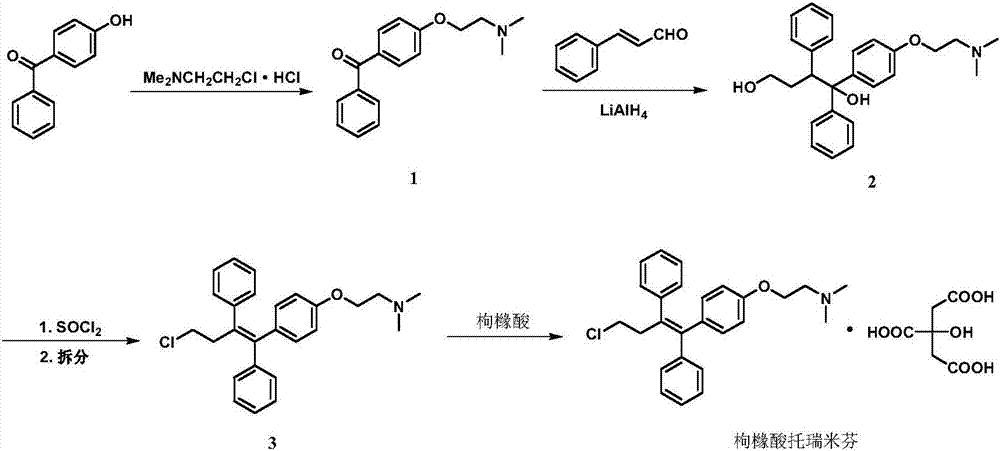
[0031] In a 10L three-necked flask equipped with mechanical stirring, add 400 g of 4-hydroxybenzophenone, 696 g of potassium carbonate, 5.4 kg of toluene, and 140 g of 95% industrial ethanol, and stir at 53-58° C. for 1 hour.
[0032] After cooling to below 40°C, 436 g of N,N-dimethylaminochloroethane hydrochloride was added, and the temperature was raised to 80-85°C to react for 4 hours.
[0033] Cool to below 40°C, add 2.4kg of drinking water, stir, separate layers, and discard the lower aqueous phase.
[0034] The oil phase was washed three times with 4.6 liters of 4% sodium hydroxide solution, and the 4-hydroxybenzophenone disappeared as detected by TLC; and then washed twice with 2 liters of drinking water until the pH was 8.
[0035] The obtained product was distilled off the solvent under reduced pressure at 55-60°C to obtain 521 g of a light yellow oil, and after cooling, a light yellow solid was obtained, namely 4-[2-(N,N-dimethylamino)ethoxy]diphenyl Methanone; the yi...
Embodiment 2
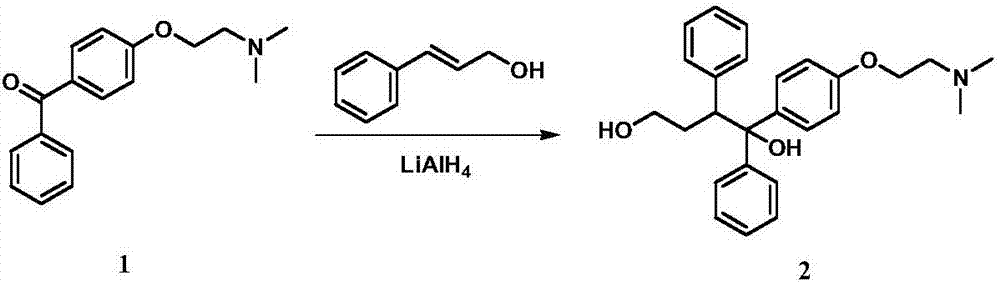
[0040] Throw 1.2kg of tetrahydrofuran and 54g of tetrahydrofuran into a 5L three-necked bottle, and control the temperature at 20-28°C. Slowly add the tetrahydrofuran solution of cinnamaldehyde (containing 318g of cinnamaldehyde and 960g of tetrahydrofuran) dropwise. Stir for 45 minutes. Then add the tetrahydrofuran solution of 4-[2-(N,N-dimethylamino)ethoxy]benzophenone prepared in Example 1 at this temperature (containing 465g4-[2-(N,N-dimethyl amino)ethoxy]benzophenone, 1.2 kg tetrahydrofuran). After the addition is complete, stir at 38-43°C for 1 hour.
[0041] Transfer the reaction solution to a 20-liter glass reactor, add 7 L of tetrahydrofuran, slowly add 132 g of 20% sodium hydroxide solution dropwise, stir for 10 minutes, add 960 g of anhydrous sodium sulfate, and then add dropwise of 132 g of drinking water. Stir for half an hour and filter with suction. The filter cake was washed with 1 L of tetrahydrofuran. Evaporate tetrahydrofuran under reduced pressure, add ...
Embodiment 3
[0052] Put 1080kg of toluene, 28kg of 95% industrial ethanol, 140kg of potassium carbonate and 80kg of 4-hydroxybenzophenone into a 2000L glass-lined reactor, and stir for 1 hour at 53-58°C.
[0053] The temperature was lowered to below 40°C, and 87.5 kg of N,N-dimethylaminochloroethane hydrochloride was added under the protection of nitrogen. After the throwing was complete, the temperature was raised to 80-85°C and stirred for 4 hours.
[0054] Cool down to below 40°C, add 500kg of drinking water, stir for 15 minutes, and let stand to separate layers. The upper organic phase was washed three times with 900 kg of 4% sodium hydroxide aqueous solution until the 4-hydroxybenzophenone disappeared as detected by TLC; the obtained organic phase was washed twice with 800 kg of drinking water until the pH value was 9. Concentrate the solvent under reduced pressure until no fraction is distilled under the conditions of a vacuum degree of not lower than 0.085 MPa and an internal temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com