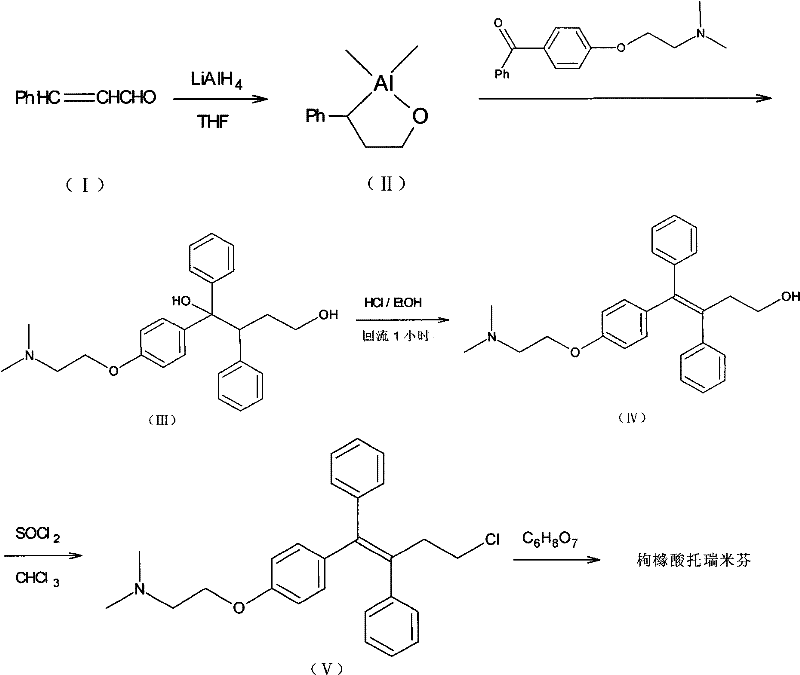
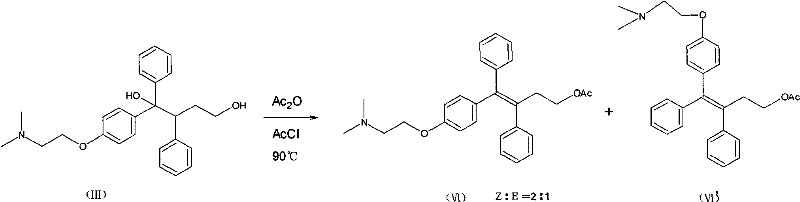
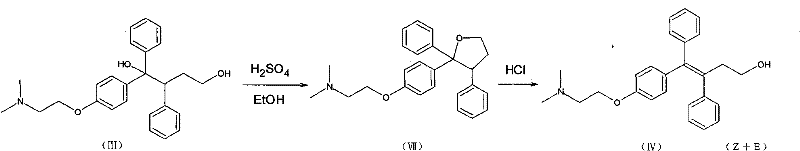
Method for realizing high-stereoselectivity synthesis of Toremifene by configuration conversion method
A technology of stereoselectivity and toremifene, applied in the field of medicine and chemical industry, achieves the effects of high stereoselectivity, high reaction yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] 8.10 g (20 mmol) of triarylbutanediol was suspended in 80 ml of concentrated hydrochloric acid, heated to 65° C., and the solid was completely dissolved, and stirring was continued for 2 hours. TLC (silica gel plate, CH 2 Cl 2 : CH 3 OH=9:1 development, UV detection) showed that the triarylbutanediol had disappeared and three spots mainly appeared. Rf0.75, accounting for about 5%, is the cyclization product (VII), Rf0.60, accounting for about 10%, is Z-type triarylbutenol; Rf0.50, accounting for about 85%, is E-type triaryl butenol.
[0035] The above mixture was gradually cooled to 40°C, and under vigorous stirring and induced by seed crystals, the reaction mixture began to become mixed and a solid precipitated. After 10 hours a large amount of solid precipitated out. TLC detection of the precipitated solid showed only the point of Rf 0.60, indicating that it was Z-type triarylbutenol hydrochloride. The TLC in the solution shows that there are almost no cyclizati...
example 2
[0038] Suspend 8.10 g (20 mmol) of triaryl butanediol in 80 ml of concentrated hydrochloric acid, stir at room temperature for 1 hour, then stir at 35°C for 15 hours, then cool to 10°C and stir for more than 2 hours, collect the resulting solid by filtration, and Suspend it in 150ml toluene, alkalinize carefully with 100ml 10% aqueous sodium hydroxide solution, separate the water layer and wash with 2×25ml water, dry over anhydrous sodium sulfate, filter and concentrate to a total volume of about 30ml, 0~5℃ After standing overnight, the precipitated white solid was collected and air-dried to obtain 6.8 g of white solid, m.p.119°C, yield 89%. The purity was 99% by HPLC.
example 3
[0040] Suspend 8.10g (20mmol) of triarylbutanediol in 40ml of concentrated hydrochloric acid, stir at room temperature for 1 hour, then stir at 35°C for 3 hours, then stir at 45°C for 7 hours, add 40ml of water to dilute for filtration, and then cool to 10°C Stir for 2 hours. By the same operation as above, 6.5 g of Z-type triaryl butenol was obtained, with a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com