Treatment of pd-l1-positive melanoma using an Anti-pd-1 antibody
a technology of pdl1 and anti-pd-1, which is applied in the field of treating pdl1-positive melanoma, to achieve the effect of reducing the size of the tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
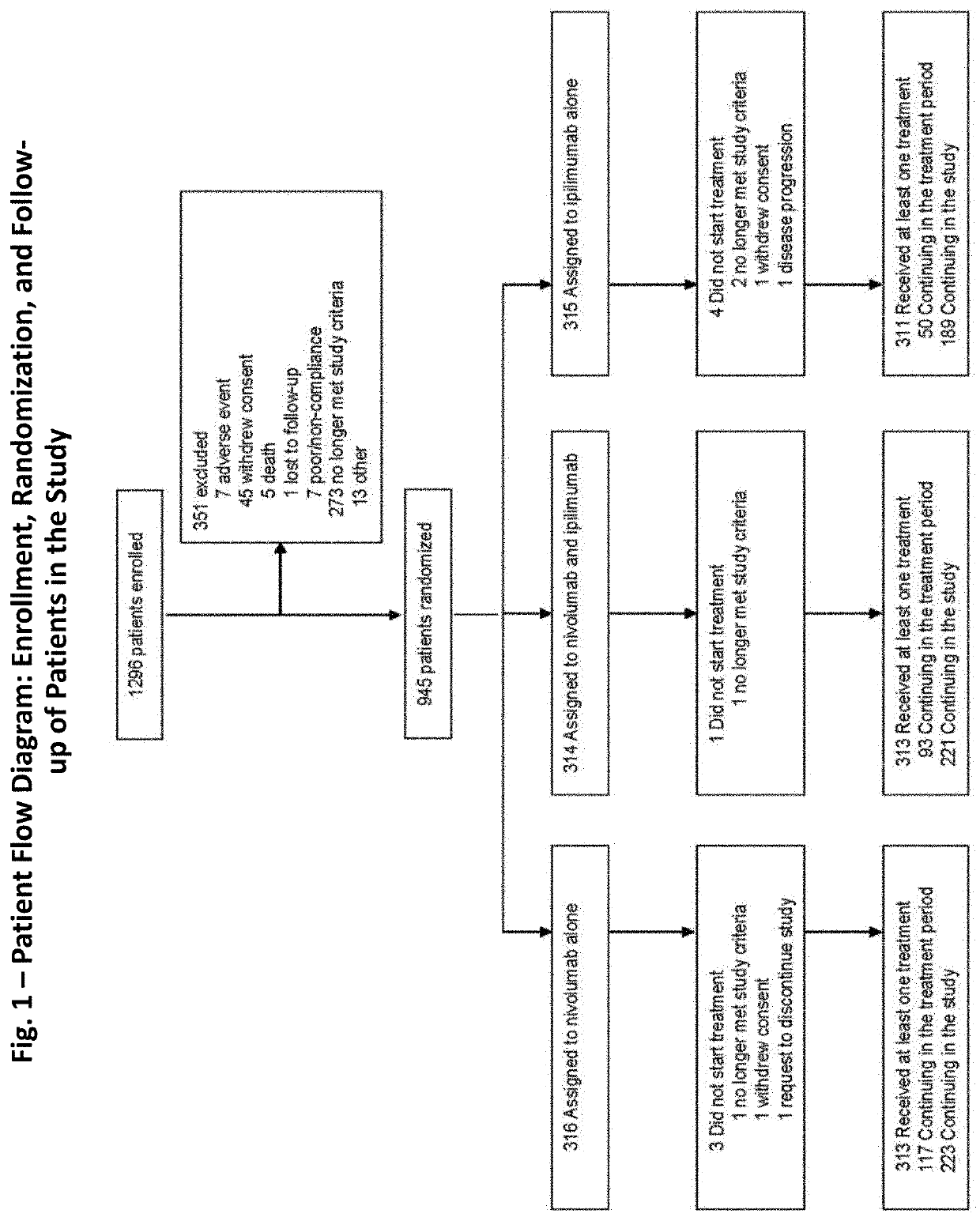
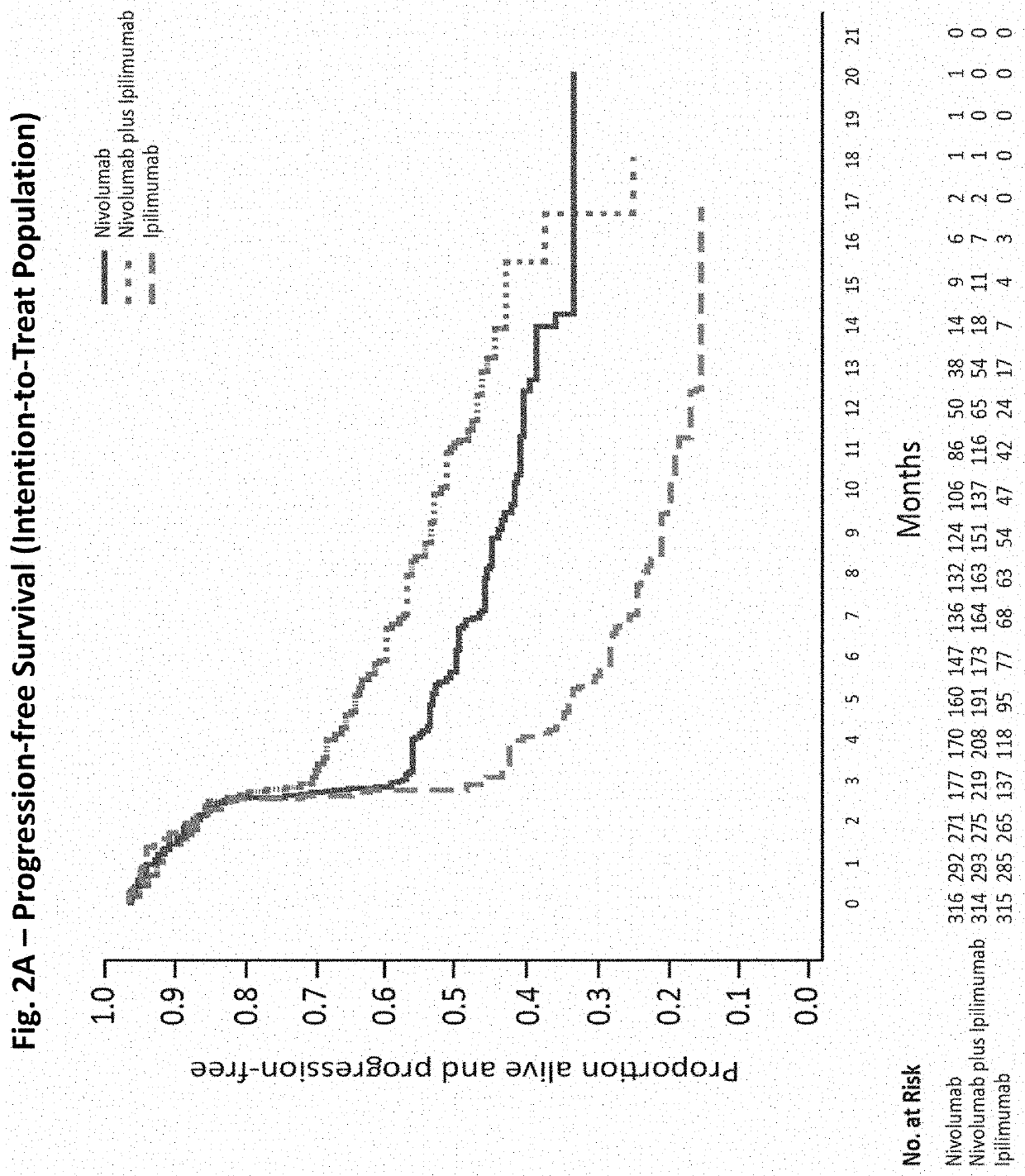
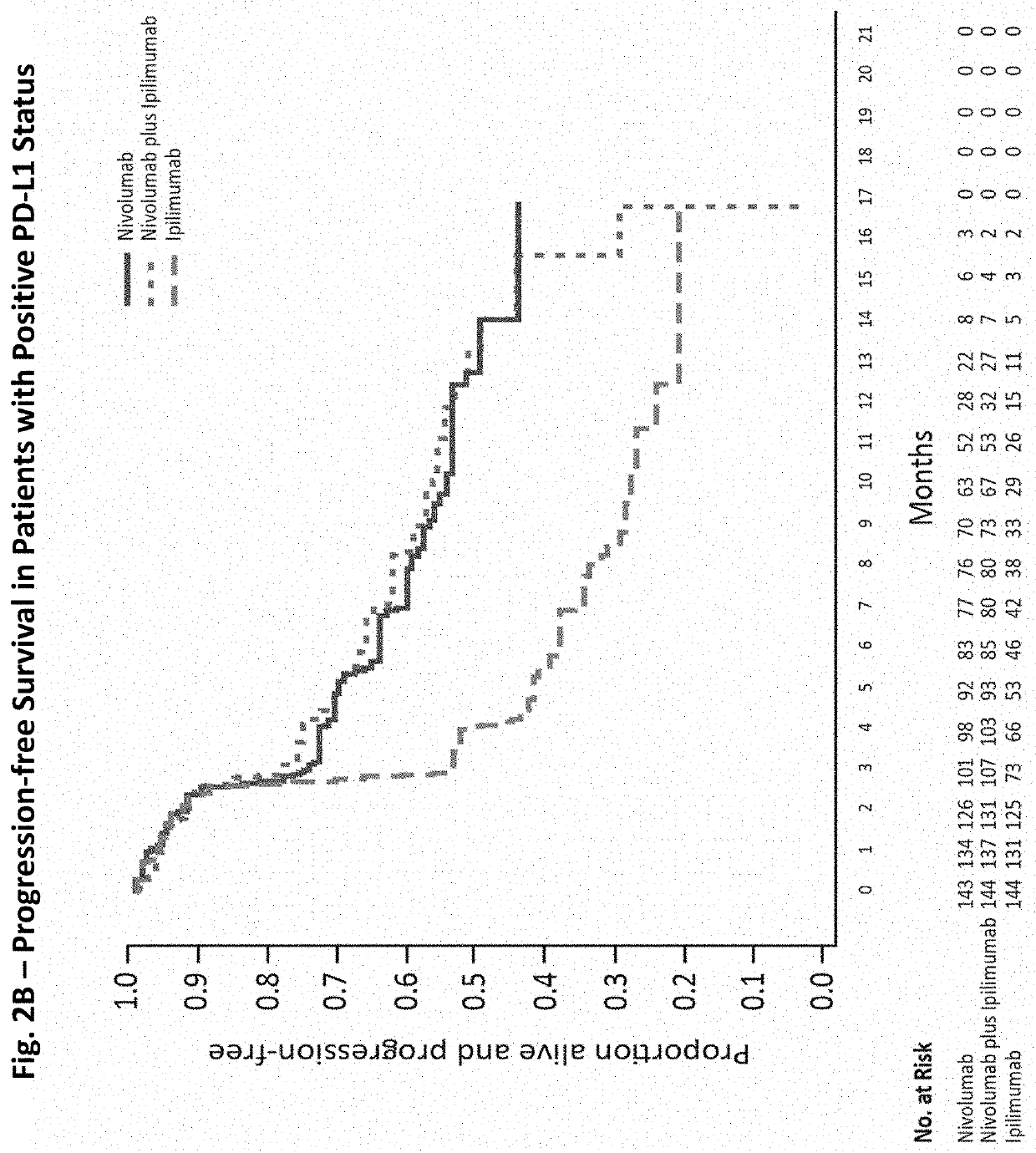
[0144]A randomized, double-blind, multicenter, phase 3 trial was conducted to evaluate the safety and efficacy of nivolumab alone or nivolumab combined with ipilimumab in comparison with ipilimumab alone in previously untreated metastatic melanoma. Patients
[0145]Eligible patients had histologically confirmed stage III (unresectable) or stage IV melanoma, and no prior systemic treatment for unresectable or metastatic melanoma. Other eligibility criteria included an age of at least 18 years, an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 (indicating no symptoms) or 1 (indicating mild symptoms), measurable disease by computed tomography or magnetic resonance imaging per RECIST v1.1, availability of tissue collected from metastatic or unresectable tumors for the assessment of PD-L1 status, and known BRAF V600 mutation status (or consent to BRAF V600 mutation testing per local standards). Key exclusion criteria were presence of active brain metastases, ocular ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com