Applications of artemisinin compounds in preparing medicines for treating liposarcoma
A liposarcoma and compound technology, applied in the field of medicine, can solve the problems of easy complications, low toxic and side effects, high treatment cost, etc., and achieve obvious therapeutic effect and high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
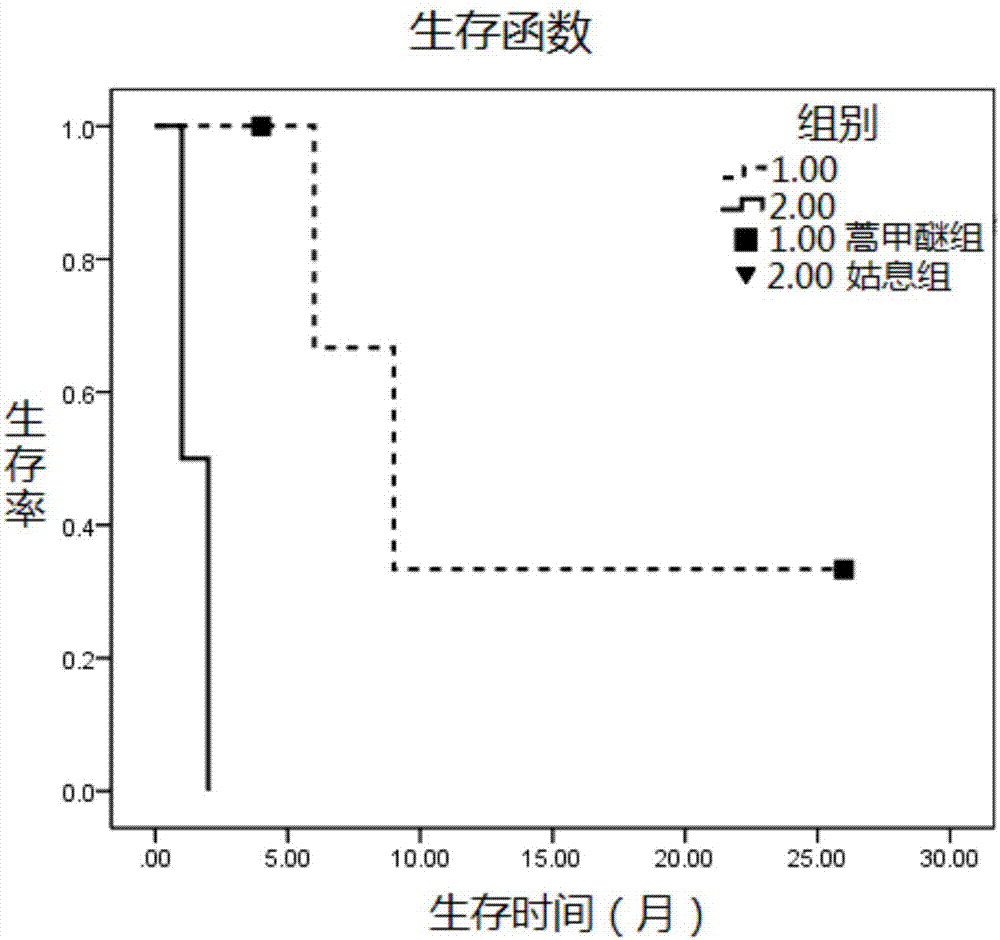
[0025] Embodiment 1, the clinical practice of artemether treatment liposarcoma
[0026] 1 Materials and methods
[0027] 1.1 Clinical data
[0028] From June 2015 to February 2017, a total of 6 patients with liposarcoma were treated in Kunming Medicine Hospital. Among them, 2 patients received palliative treatment due to economic reasons after failure of other treatments, and the remaining 4 patients received the method of this study, that is, artemether The general clinical data are shown in Table 1. Inclusion criteria of this study: Sarcoma patients who failed or refused surgery, radiotherapy and chemotherapy, and the last treatment (surgery, chemotherapy, radiotherapy) was completed for at least 1 month. Age ≥18 years; histologically confirmed liposarcoma. Other inclusion criteria include: expected survival of at least >3 months; normal or nearly normal blood tests and liver and kidney functions. All patients voluntarily received artemether treatment and signed an infor...
Embodiment 2
[0066] Embodiment 2, the preparation of artemisinin compound tablet
[0067] The artemisinin compound is mixed with conventional auxiliary materials, and the artemisinin compound tablet is prepared according to a conventional method.
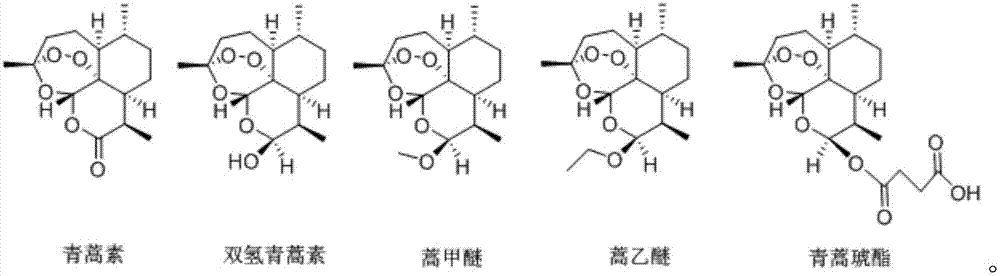
[0068] Wherein, the artemisinin compounds are as follows:
[0069]
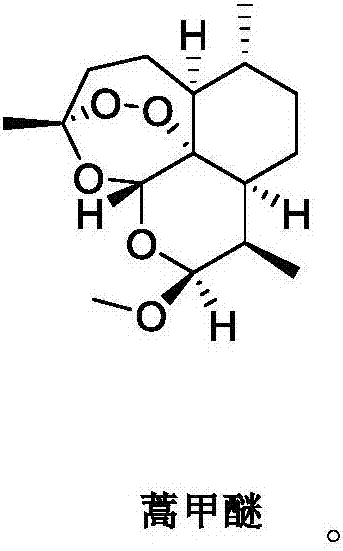
[0070] Preferably, the artemisinin compound is artemether, as shown below:
[0071]
Embodiment 3
[0072] Embodiment 3, the preparation of artemisinin compound oral liquid
[0073] The artemisinin compound is mixed with conventional auxiliary materials, and the artemisinin compound oral liquid is prepared according to a conventional method.
[0074] Wherein, the artemisinin compounds are as follows:
[0075]
[0076] Preferably, the artemisinin compound is artemether, as shown below:
[0077]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com