Combination anticoagulant therapy with a compound that acts as a factor xa inhibitor
a technology of factor xa inhibitor and anticoagulant therapy, which is applied in the direction of extracellular fluid disorder, antibody medical ingredients, metabolic disorders, etc., can solve the problems of insufficient therapeutic benefit, inability to combine with an antiplatelet agent at a fixed dose, and current anticoagulant therapies are not suitable for combination therapy, so as to achieve superior efficacy or safety, the effect of predicting the level of anticoagulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compound A
[0209]
Step 1:
[0210]5-Methoxy-2-nitrobenzoic acid (1) (25.0 kg, 1.0 eq), 2-amino-5-chloropyridine (2) (16.3 kg, 1.0 eq), and acetonitrile (87.5 kg, 3.5 parts) were charged to a 380 L GLMS reactor. The reaction mixture was adjusted to 22° C. (19 to 25° C.) and anhydrous pyridine (30.0 kg, 3.0 eq) was added. The pump and lines were rinsed forward with acetonitrile (22.5 kg, 0.9 parts), and the reactor contents were adjusted to a temperature of 19-22° C. Phosphorous oxychloride (23.3 kg, 1.20 eq) was charged to the contents of the reactor via a metering pump, while maintaining a temperature of 25° C. (22-28° C.). The metering pump and lines were rinsed forward with acetonitrile (12.5 kg, 0.5 parts), while keeping the temperature at 25° C. (22-28° C.). The reaction mixture normally turned from a slurry to a clear solution after the addition of about ⅓ of the POCl3. At the end of the addition, it became turbid. After complete addition, the reaction mixture was agi...
example 2
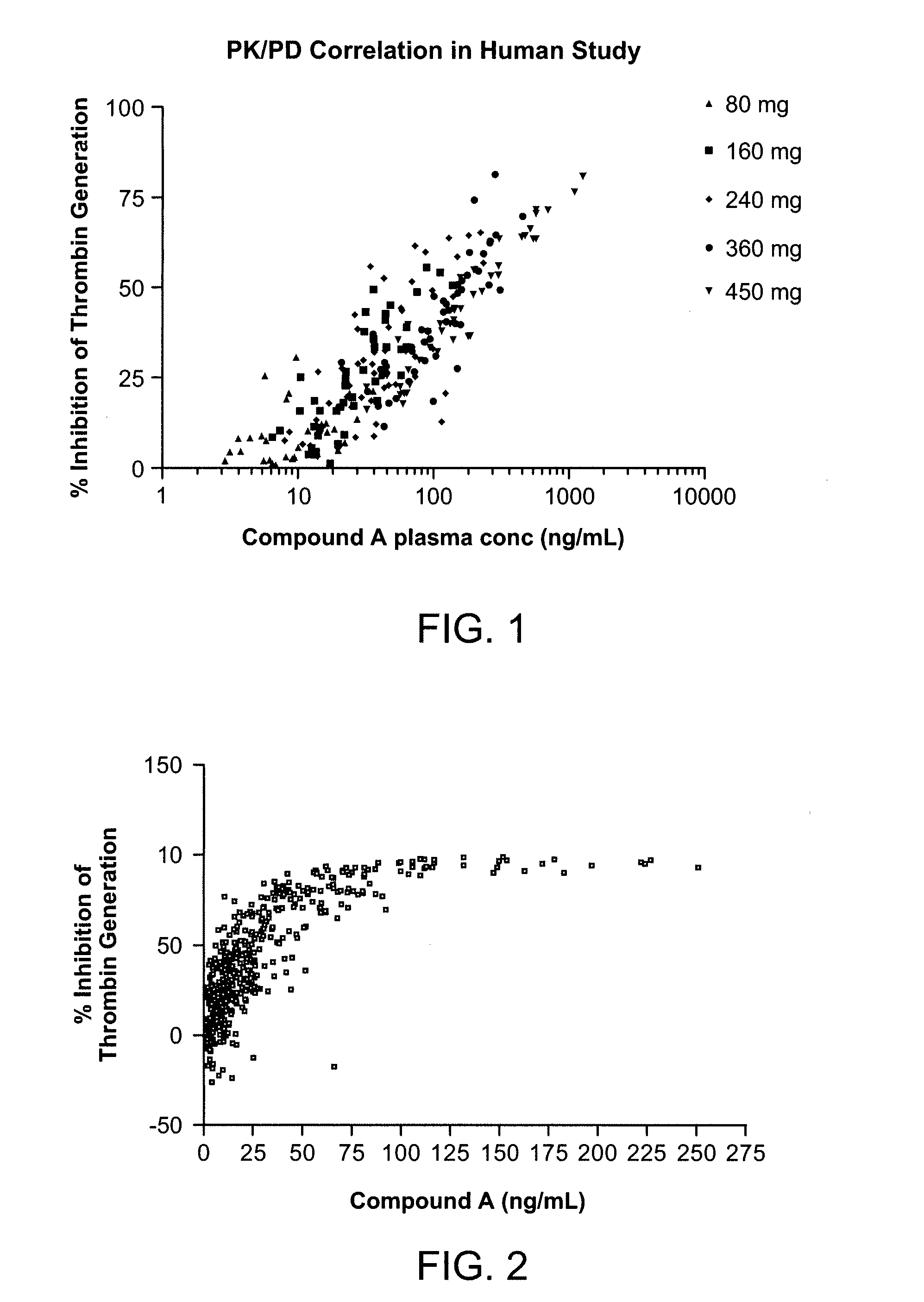
Thrombin Generation Inhibition Assay
[0214]In this method, human plasma samples containing the peptide gly-pro-arg-pro (Pefabloc FG, Centerchem) as an anticlotting agent, were treated with tissue factor (Innovin, Dade Behring) to initiate the generation of thrombin. After 10 min, the reaction was stopped by addition of EGTA. A chromogenic peptide substrate (Spectrozyme TH, American Diagnostica) specific for thrombin was added to measure the activity of thrombin generated during the tissue factor treatment period. After allowing the substrate cleavage reaction to proceed for 2 min, the samples were quenched with glacial acetic acid. The plasma samples were analyzed in triplicate in a 96-well plate format. Control samples containing pooled platelet poor plasma with or without added Compound A were assayed in quadruplicate on each plate. The control samples were used to establish plate acceptance criteria. Absorbance of each sample well was measured at 405 nm.
[0215]Thrombin generation (...
example 3
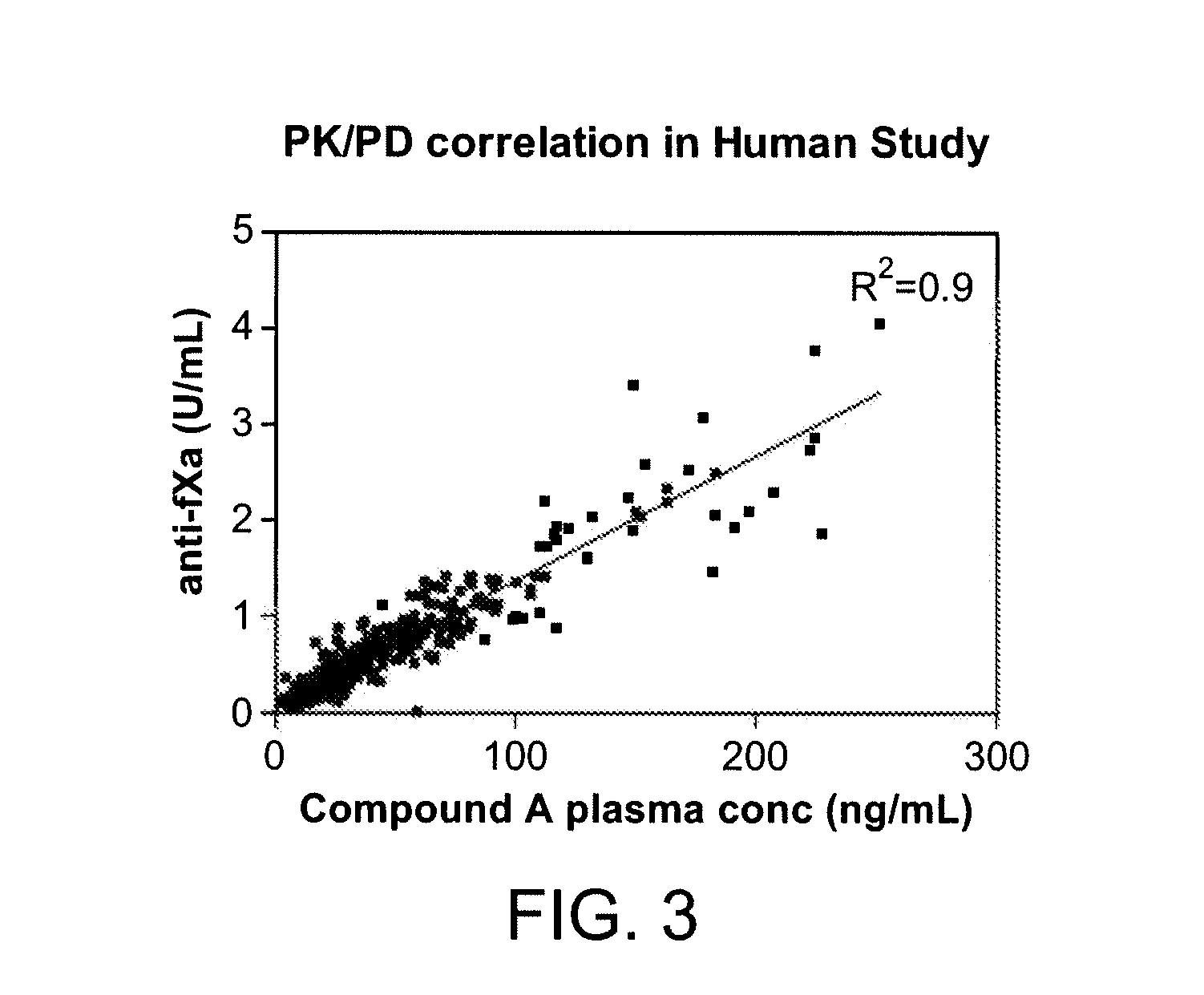
Anti-Factor Xa Unit Assay
[0218]The anti-factor Xa (anti-fXa) assay was adapted from a commercial kit (Diapharma COATEST LMW Heparin) and modified to a 96-well format. A low molecular weight heparin, dalteparin, included in the kit was used to construct a standard curve according to the manufacture's instruction using pooled platelets poor plasma. All samples and standards were assayed in duplicate. The limit of quantitation was 0.05 U / mL and results below 0.05 U / mL were reported as below the limit of quantitation. The upper limit of measurement for the plate assay was 1.0 U / mL and samples with anti-factor Xa units higher than 1.0 U / mL were re-assayed after dilution. The coefficient of variation (CV) for both standards and clinical samples were 10%.
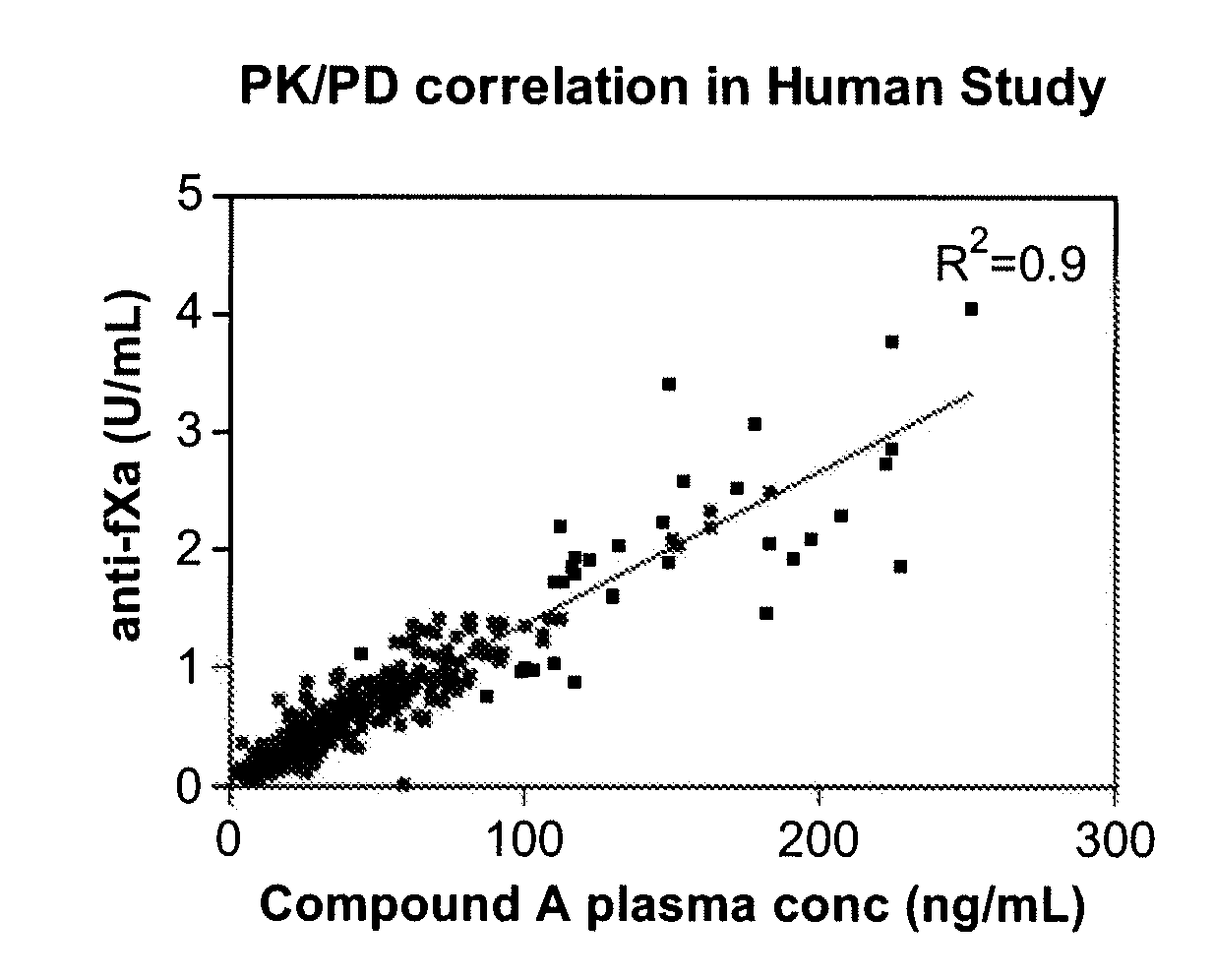
[0219]FIG. 3 shows the anti-factor Xa units generated by plasma samples from healthy volunteers who were administered multiple ascending doses of Compound A.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com