Warfarin individual anticoagulant pharmacogenomics detection kit suitable for Chinese population
A detection kit and genomics technology, applied in the field of kits, can solve the problems of narrow treatment window, bleeding side effects, and differences, and achieve the effect of simple preparation and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Collection of Warfarin Treatment Cases in the Chinese Population and Collection of Whole Blood DNA
[0030] 1. Collection and grouping of warfarin stable treatment cases: collect Chinese patients who have been treated with warfarin stable anticoagulant therapy for at least three months, exclude patients younger than 18 years old, and those diagnosed with cancer, liver and kidney insufficiency, or Patients with congestive heart failure.
[0031] 2. Collect clinical data related to warfarin treatment and dose, including basic clinical information of the patient (age, sex, height, weight, etc.), medical history related to warfarin dose and side effects, indications for warfarin treatment, The target prothrombin international normalized ratio (PT-INR) value, and the concomitant medication that interacts with warfarin, etc. When the INR reached a stable value for at least 3 months, the average daily dose of warfarin during stable therapy was recorded. Please ref...
Embodiment 2
[0036] Example 2: Detection of genetic variants using sanger sequencing
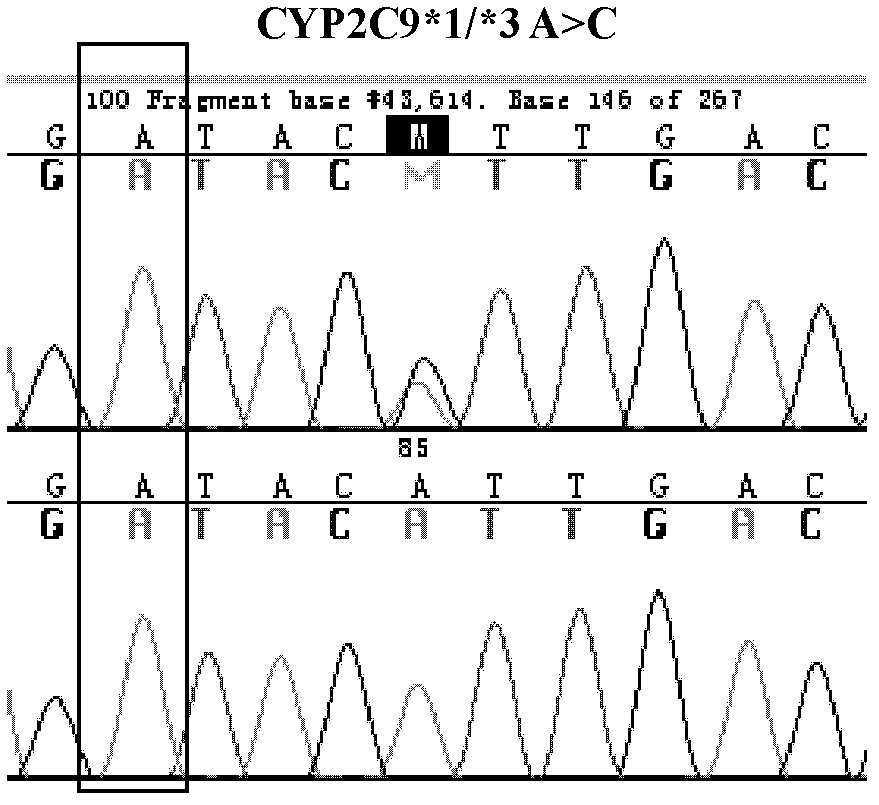
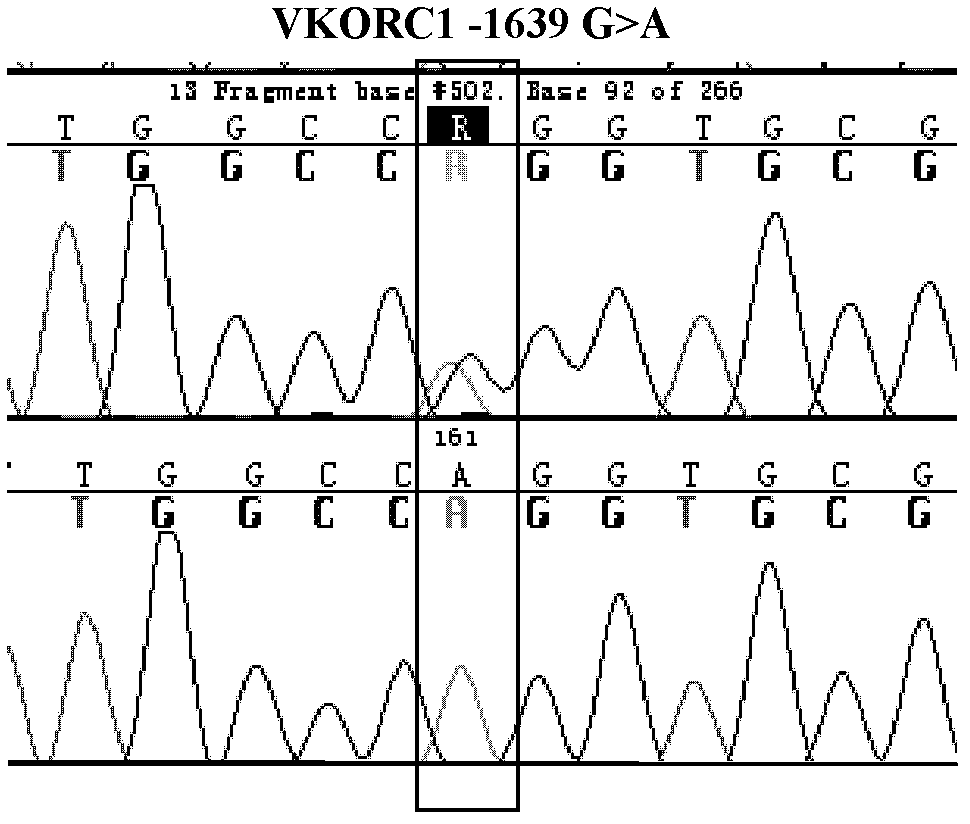
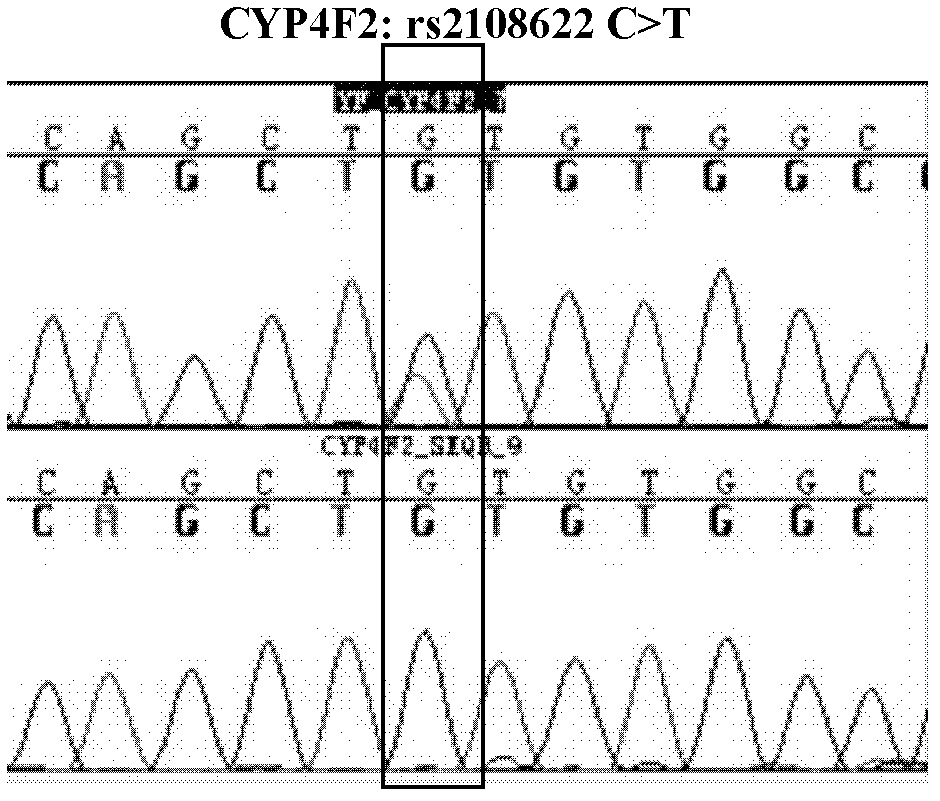
[0037] Design synthetic primers at both ends of the nucleotide sequence fragment at the position of the gene variant to be detected, amplify the fragment by polymerase chain reaction (PCR), and then apply Sanger sequencing to sequence and analyze the fragment, and pass the reference Sequence comparison to find the genetic variant of the site to be tested.
[0038] 1. Design synthetic primers to amplify the site of the target gene fragment and the surrounding known DNA sequence of the mutant CYP2C9*3, VKORC1 (-1639G>A) and CYP4F2 (rs2108622).
[0039] Amplification primers:
[0040] CYP2C9*3 (303bp)
[0041] Upstream primer: 5'-CCCCTGAATTGCTACAACAAA-3' (SEQ ID NO: 1)
[0042] Downstream primer: 5'-GGGACTTCGAAAACATGGAG-3' (SEQ ID NO: 2)
[0043] VKORC1(-1639G>A)(290bp)
[0044] Upstream primer: 5'-GCCAGCAGGAGAGGGAAATA-3' (SEQ ID NO: 3)
[0045] Downstream primer: 5'-AGTTTGGACTACAGGTGCCT-3' (SEQ ID NO...
Embodiment 3
[0068] Embodiment 3: the preparation and use method of kit
[0069] 1. Components of the kit
[0070] The main components of this kit are amplification primers and sequencing primers described in Example 2, PCR reaction reagents: HotStarTaq Master Mix Kit, sequencing reagents: Terminator v3.1Cycle Sequencing Kit, which can also include instructions for describing the pharmacogenomics dose prediction model of warfarin suitable for the Chinese population or / and computer-readable storage with the pharmacogenomics dose prediction model of warfarin recorded for the Chinese population medium.
[0071] 2. Storage conditions of the kit
[0072] The dry powder primers in the kit can be stored at room temperature for 2 months, and can be stored at -20°C for a long time. Dry powder primers can be stored stably at -20°C for 6 months after dissolving, and can be stored at room temperature for 120 hours when used frequently in a short period of time. HotStarTaq Master Mix Kit-20℃ can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com