A kind of olanzapine related substance and its preparation method and high performance liquid chromatography analysis method
A technique for high performance liquid chromatography and related substances, applied in the fields of high performance liquid chromatography analysis, olanzapine related substances and their preparation, can solve problems such as difficulty in isocratic elution separation, and achieve the effects of high sensitivity and variety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation and refining of embodiment 1 olanzapine
[0043] Add 19.99 g (0.075 mol) of 4-amino-2-methyl-10H-thieno[2,3-b][1,5]-benzodiazepine hydrochloride, N- Methylpiperazine 45.01g (0.45mol), nitrogen gas and stirring, heated to reflux reaction for 2h. The temperature was lowered, and more than 75% of excess N-methylpiperazine was recovered by distillation under reduced pressure to obtain a crude olanzapine solid.
[0044] Add 60ml of ethanol to the three-necked flask, and heat to reflux to completely dissolve the solid. Then, the ethanol solution in which olanzapine was dissolved was poured into 400 ml of water under stirring, and a yellow powder solid was precipitated, which was filtered and dried to obtain 23.37 g of olanzapine product, with a yield of 99.7% and a purity of 99.0% (HPLC).
[0045] Refining of olanzapine: transfer 23.37g olanzapine product into a 500ml single-necked bottle, add 250ml ethanol, stir, and heat to reflux to completely dissolve th...
Embodiment 2
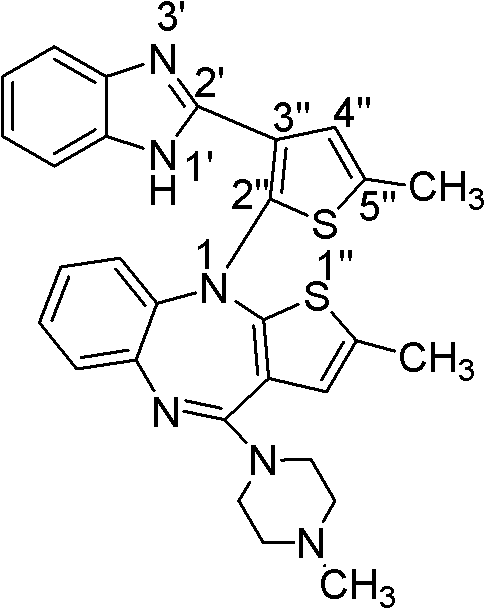
[0046] Example 2 Olanzapine-related substances 1-(2-(5-methylthiophen-3-yl)-1H-benzimidazolyl)-2-methyl-4-(4-methyl-1-piperazine base)-10H-thieno[2,3-b][1,5]-benzodiazepine preparation
[0047] Concentrate the olanzapine recrystallization mother liquor obtained in Example 1 to obtain 7.00 g of brown powder, mix well with 6.20 g of silica gel; use silica gel column chromatography, apply dry method, and use dichloromethane-methanol 98:2 as eluent For elution, dichloromethane-methanol-diethylamine 95:5:0.5 was used as the developing solvent to detect the effluent by thin-layer chromatography; the effluent containing Rf of 0.3 was combined and concentrated, and dichloromethane-methanol 97:3 was used as the The eluent was purified by column chromatography and concentrated to obtain 0.98 g of off-white blocky solid. The structure of this compound was determined by X-ray single crystal diffraction, see above for the single crystal structure.
Embodiment 3
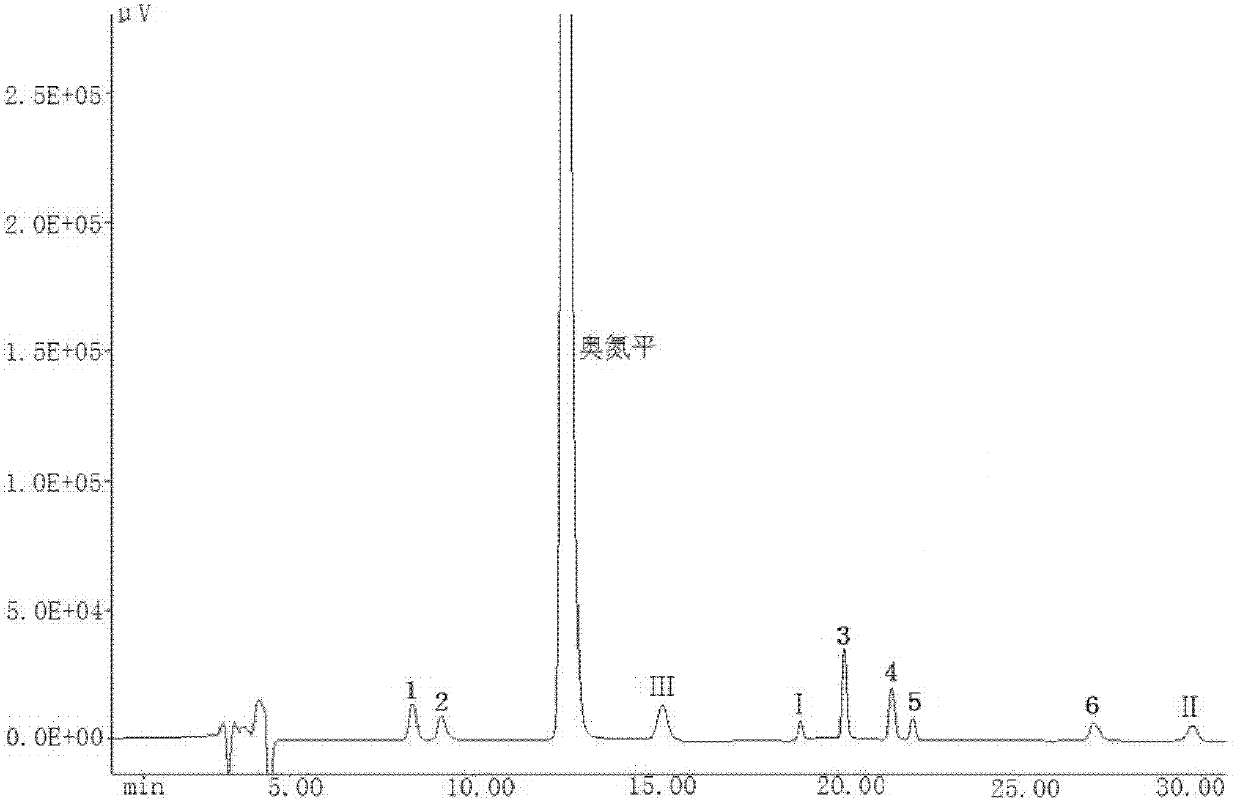
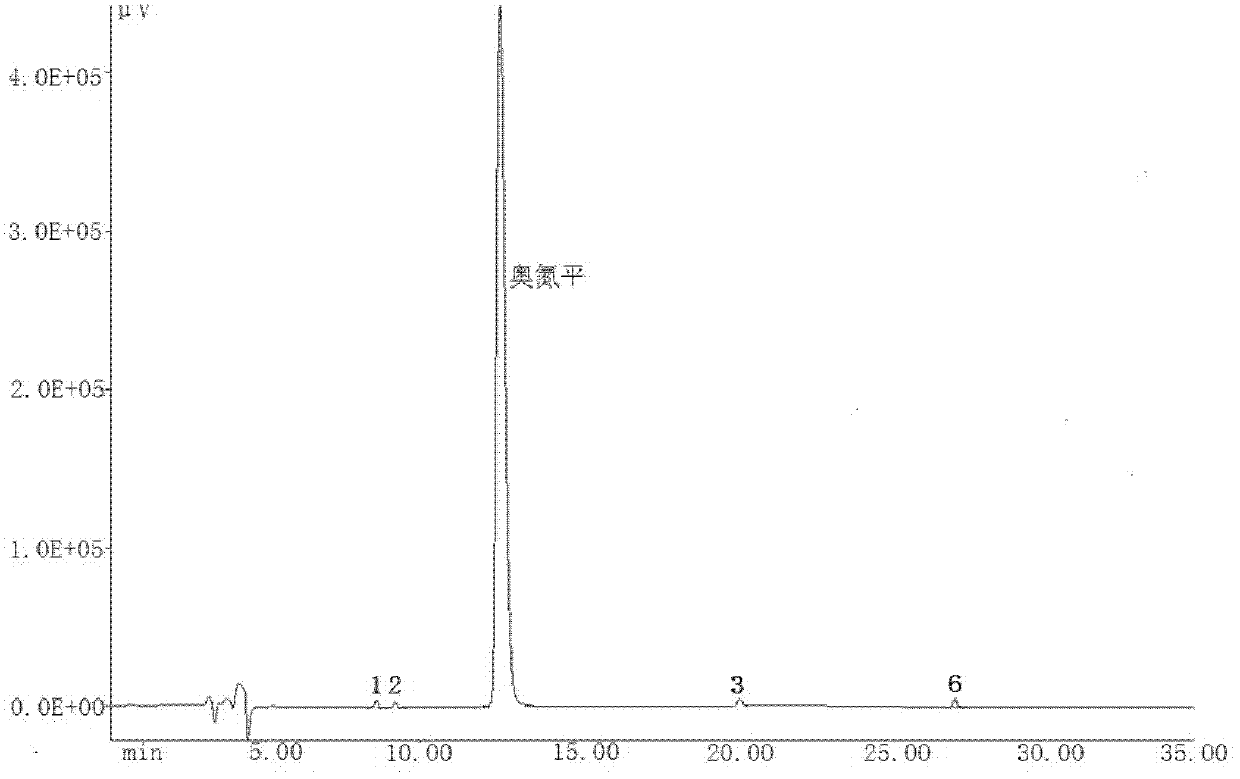
[0048] The high-performance liquid chromatography analysis of embodiment 3 olanzapine and 9 related substances
[0049]The 9 related substances are: 2-methyl-4-(1-piperazinyl)-10H-thieno[2,3-b][1,5]-benzodiazepine (1), 2- Methyl-4-(4-methyl-N-oxo-1-piperazinyl)-10H-thieno[2,3-b][1,5]-benzodiazepine (2), 4-amino-2-methyl-10H-thieno[2,3-b][1,5]-benzodiazepine hydrochloride (III), 2-amino-5-methylthiophene-3 - nitrile (I), 2-methyl-4-oxo-10H-thieno[2,3-b][1,5]-benzodiazepine (3), dimer-2-methyl -4-(1-piperazinyl)-10H-thieno[2,3-b][1,5]-benzodiazepine (4), 1-(5-methyl-thiophene-2- Base)-1H-benzimidazol-2(3H)-one (5), 1-(2-(5-methylthiophen-3-yl)-1H-benzimidazolyl)-2-methyl-4 -(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]-benzodiazepine (6), 2-(2-nitroanilino )-5-methylthiophene-3-carbonitrile (II), the structural formula is as follows.
[0050]
[0051] Instrument and analysis conditions - Japan Spectroscopic JASCO HPLC system, including: PU-1580 double pump, UV-1575 ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com