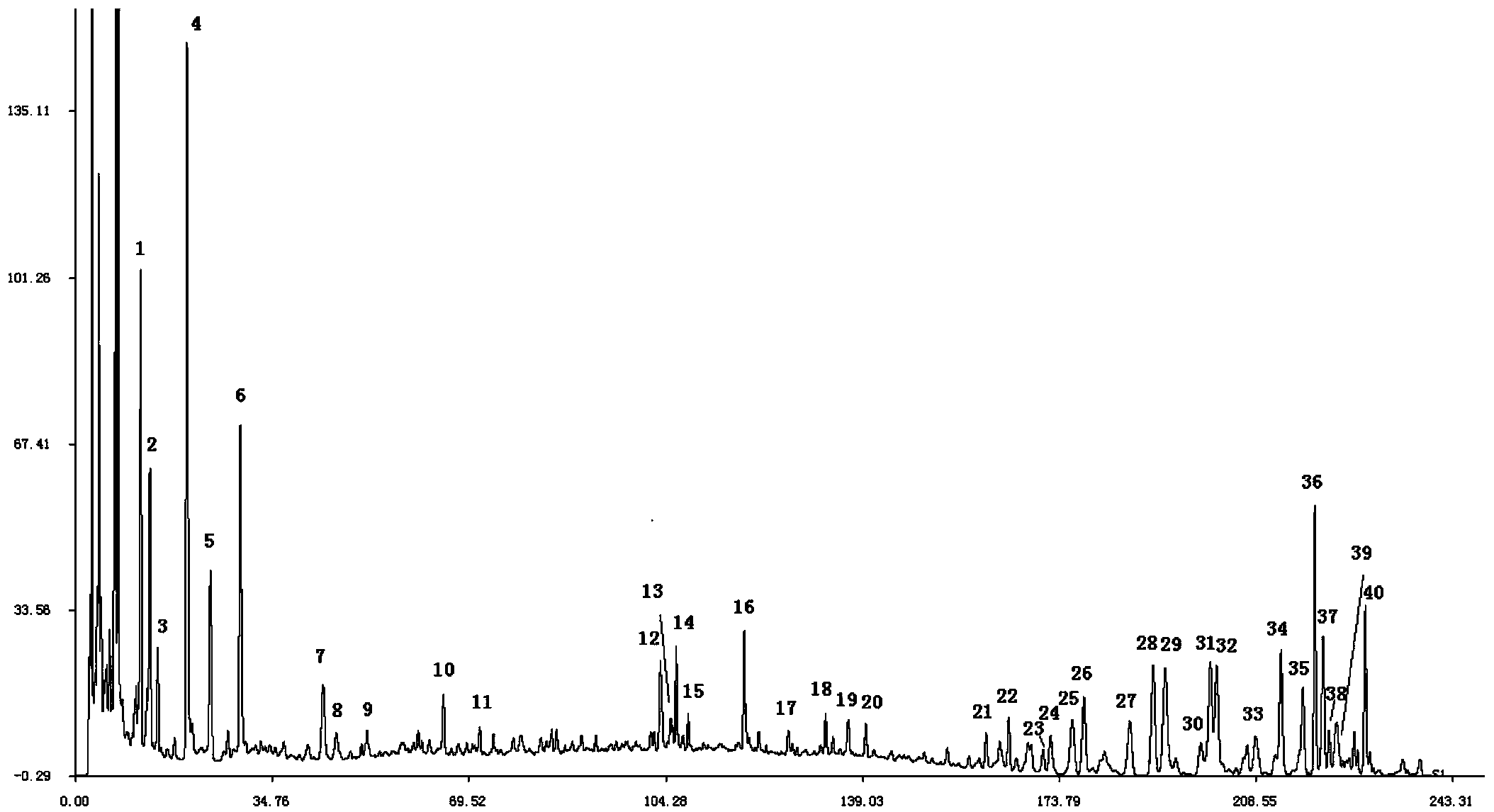
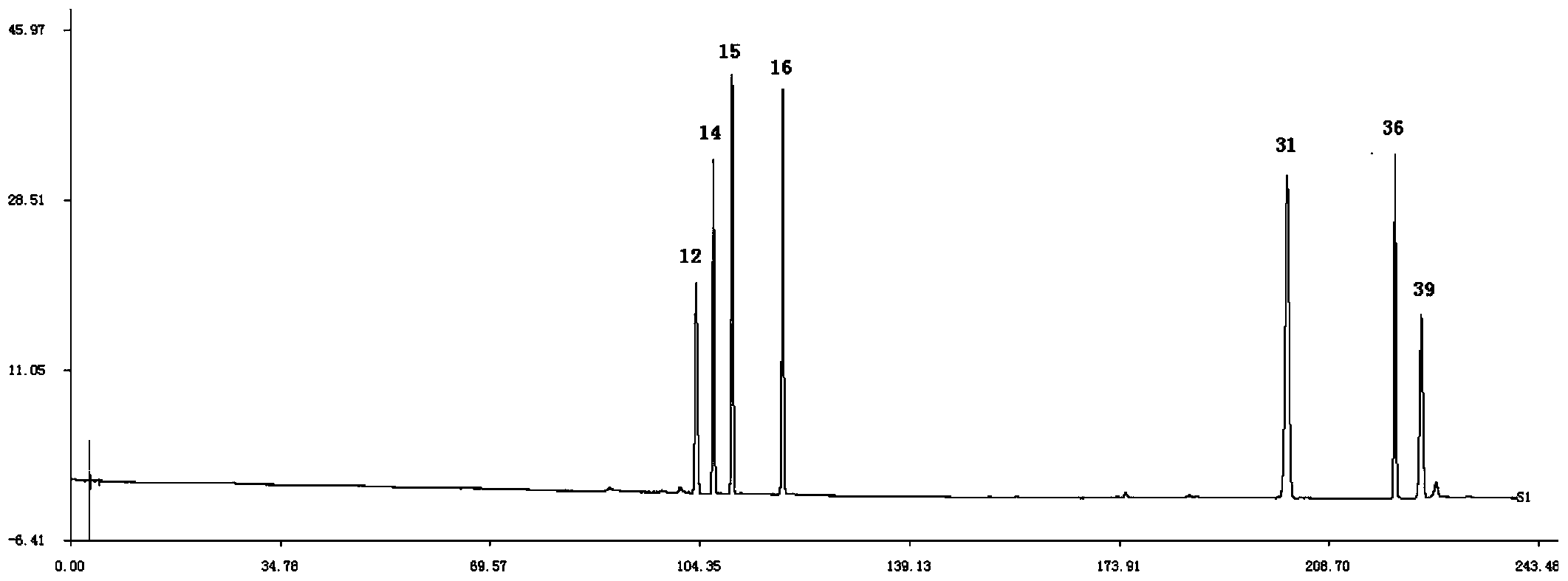
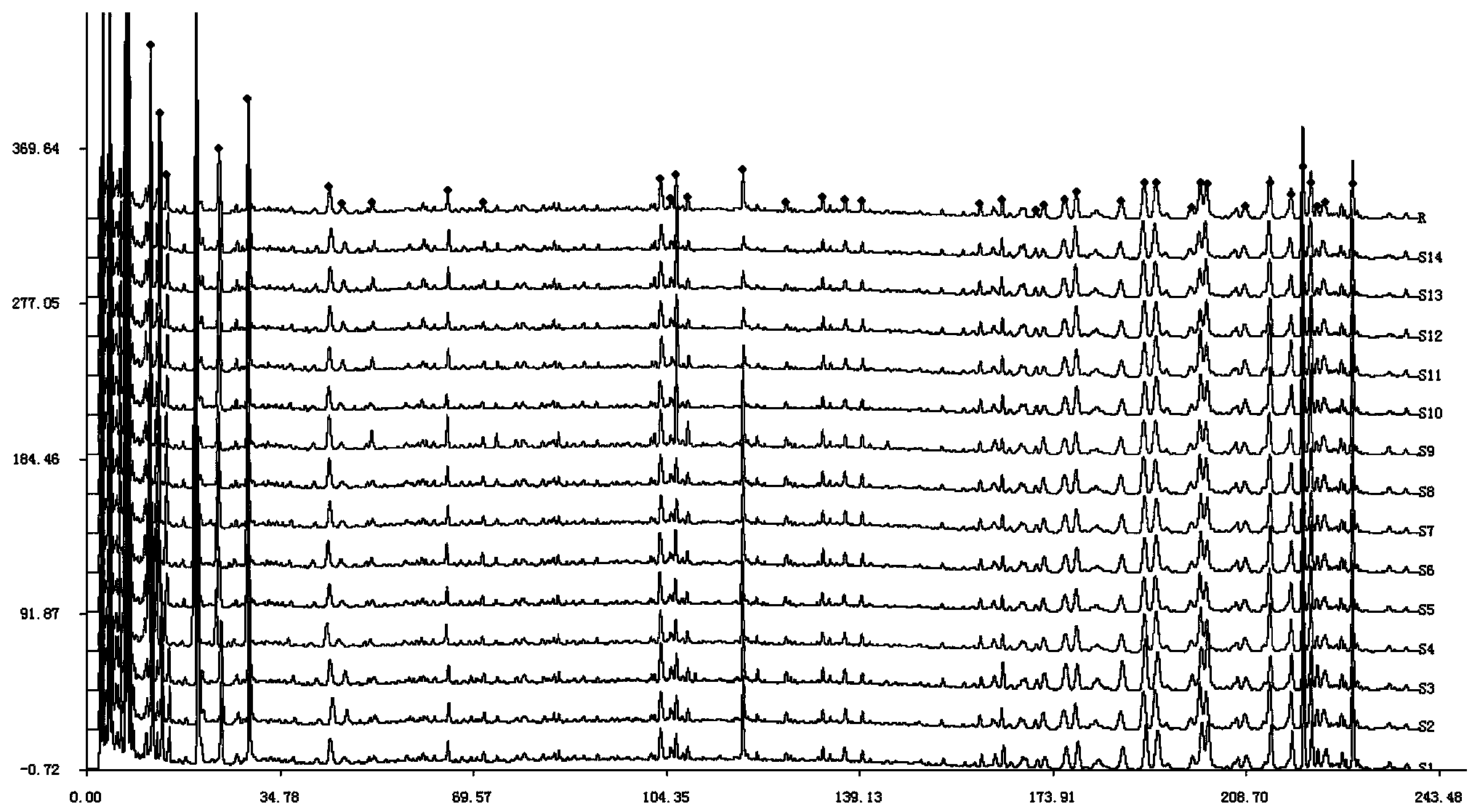
High performance liquid chromatography (HPLC) standard fingerprint spectrum of lucid ganoderma capsule preparation and construction method and application of standard fingerprint spectrum
A standard fingerprint and fingerprint technology, applied in the field of drug analysis, can solve problems such as difficulty in comprehensively monitoring the production process stability of finished products and intermediate products, lack of quality control methods, and inability to fully reflect characteristics, so as to avoid singleness and one-sidedness, Simple method, good specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Instruments and reagents
[0057] Instrument: Agilent1200 high performance liquid chromatography (quaternary pump, column thermostat, DAD detector and Agilent1200 chromatographic workstation), American Agilent Company; laboratory-specific ultrapure water machine, Chongqing Lidi Modern Water Technology Equipment Co., Ltd.; BP211D type Analytical balance, German Sartorius company; HWS24 electric heating constant temperature water bath, Shanghai Yiheng Technology Co., Ltd.; KQ-400KPE ultrasonic cleaning instrument, Kunshan Ultrasonic Instrument Co., Ltd.
[0058] Control substance:
[0059]
[0060] Ganoderma Lucidum Capsule: a product of Infinitus (China) Co., Ltd., it is a capsule. Ganoderma lucidum preparation is mainly made of medicinal materials such as Ganoderma lucidum, Atractylodes macrocephala, Polygonatum, Lycium barbarum, Poria cocos, Radix Ophiopogon japonicus, Cuscuta seed, Schisandra chinensis, Coix seed, and licorice.
[0061] Control raw materials: ...
Embodiment 2
[0109] 1, instrument, reagent are the same as embodiment 1.
[0110] 2. Determination conditions of high performance liquid chromatography
[0111] Chromatographic column: Kromasil100-5C 18 (4.6×250mm i.d., 5μm); mobile phase: acetonitrile as mobile phase A, and 0.15% by volume phosphoric acid aqueous solution as mobile phase B; detection wavelength: 252nm; column temperature: 30°C; flow rate: 1.0mL / min. Adopt the gradient elution mode identical with embodiment 1:
[0112] 3. Establishment of HPLC fingerprints
[0113] 1) Preparation of test sample and reference substance
[0114] Preparation of Ganoderma lucidum test solution: Take 0.5 g of the content of Ganoderma lucidum capsule, accurately weigh it, place it in a conical flask with a stopper, add 30 mL of methanol precisely, seal it tightly, weigh it, and ultrasonically treat it for 30 minutes (power 500W, frequency 40KHz), let stand to cool, then weighed, make up for the lost weight with methanol, shake well, filter...
Embodiment 3
[0119] 1, instrument, reagent are the same as embodiment 1.
[0120] 2. Determination conditions of high performance liquid chromatography
[0121] Chromatographic column: Kromasil100-5C 18 (4.6×250mm i.d., 5μm); mobile phase: acetonitrile as mobile phase A, and 0.05% phosphoric acid aqueous solution as mobile phase B; detection wavelength: 252nm; column temperature: 25°C; flow rate: 1.0mL / min. The same gradient elution method as in Example 1 was adopted.
[0122] 3. Establishment of HPLC fingerprints
[0123] 1) Preparation of test sample and reference substance
[0124] Preparation of Ganoderma lucidum test solution: Take 2.0 g of Ganoderma lucidum capsule content, accurately weighed, place in a conical flask with a stopper, add 40 mL of methanol precisely, seal the plug, weigh, and ultrasonically treat for 60 minutes (power 500W, frequency 40KHz), let stand to cool, then weighed, make up for the lost weight with methanol, shake well, filter, discard the initial filtra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com