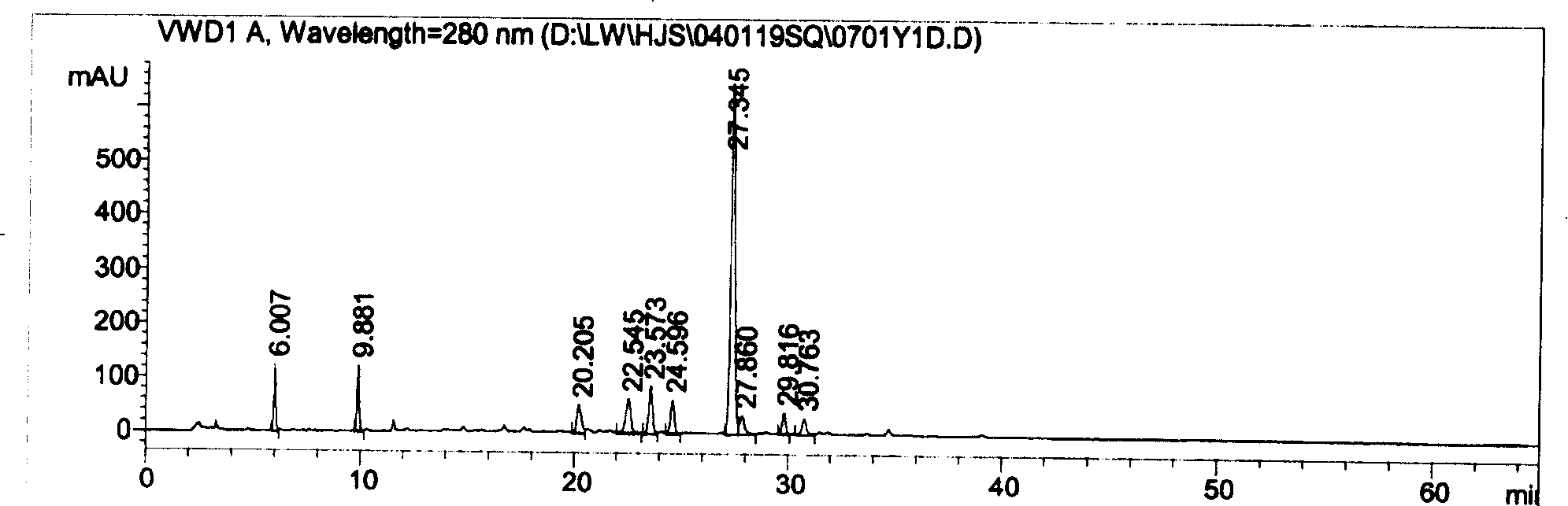
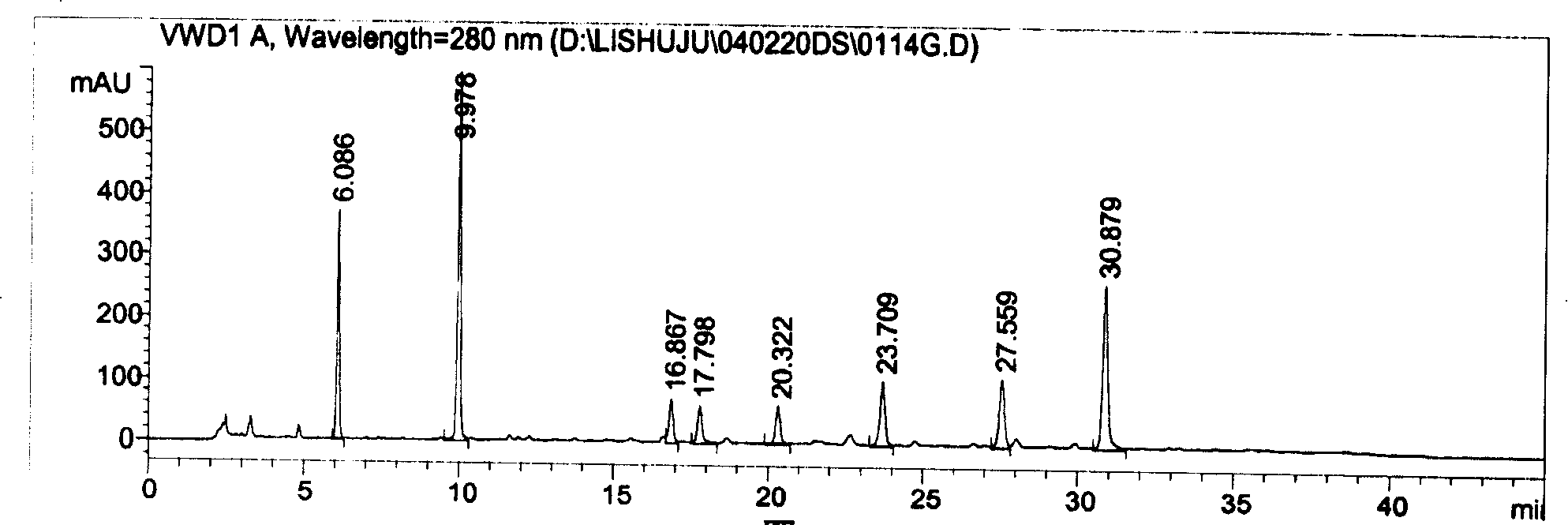
Quality control of compound Danshen root drops
A quality control method, the technology of compound salvia miltiorrhiza, applied in the direction of pill delivery, measuring device, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Embodiment one (preparation example)
[0145] Weigh 41.06g of Salvia miltiorrhiza and 8.03g of Panax notoginseng, add water to decoct twice, measure water 4 times for 2 hours for the first time, measure water 3 times for 1 hour for the second time, filter, combine the filtrates, concentrate to a specific gravity of 1.19-1.20( 75±1°C), add ethanol with a concentration of about 90% until the ethanol content is 65% (20°C), let it stand for 12 hours, separate the supernatant, recover the ethanol, concentrate until the relative density of the liquid is 1.37 (55-60 ℃), to obtain the Radix Notoginseng Extract. Take the above-mentioned Danshen Sanqi extract and 0.46g of borneol, mix it with 18g of polyethylene glycol-6000 evenly, heat it to a temperature of 85°C, and after 80 minutes of mixing, move it to a dripping tank of a dripping machine whose tank temperature is kept at 86°C . Drop the drug liquid into liquid paraffin at 8°C, take out the dropping pills, remove the oil,...
Embodiment 2
[0146] Embodiment two (preparation example)
[0147] Weigh 59.36g of Salvia miltiorrhiza and 6.38g of Panax notoginseng, add water to decoct twice, measure 4 times of water for 2.5 hours for the first time, and measure 3 times of water for 1.5 hours for the second time, filter, combine the filtrates, and concentrate until the specific gravity is 1.19-1.20( 75±1°C), add ethanol with a concentration of about 85% until the ethanol content is 70% (20°C), let it stand for 10 hours, separate the supernatant, recover the ethanol, concentrate until the relative density of the liquid is 1.35 (55-60 ℃), to obtain the Radix Notoginseng Extract. Take the above-mentioned Danshen Sanqi extract and 0.34g of borneol, mix it with 23g of polyethylene glycol-6000 evenly, heat it to a temperature of 89°C, and after 100 minutes of mixing, move it to a dripping tank of a dripping machine whose tank temperature is kept at 85°C . Drop the liquid medicine into methyl silicone oil at 8°C, take out th...
Embodiment 3
[0148] Embodiment three (preparation example)
[0149] Weigh 31.12g of salvia miltiorrhiza and 9.21g of notoginseng, add 0.5% sodium hydroxide of the total amount of medicinal materials, decoct twice, the first time 4 times the amount of water for 1.5 hours, the second time 3 times the amount of water for 1.5 hours, filter, and the filtrate Combine and concentrate until the specific gravity is 1.19-1.20 (75±1°C), add ethanol with a concentration of about 88% until the ethanol content is 66% (20°C), let stand for 10 hours, separate the supernatant, reclaim the ethanol, and concentrate to The relative density of the liquid medicine is 1.40 (55-60° C.), and the extract of Danshen Sanqi is obtained. Take the above-mentioned Danshen Sanqi extract, 0.50 g of borneol, 90 g of mannitol, 15 g of edetate calcium sodium and 15 ml of distilled water, mix the above components, freeze-dry, and make a powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com