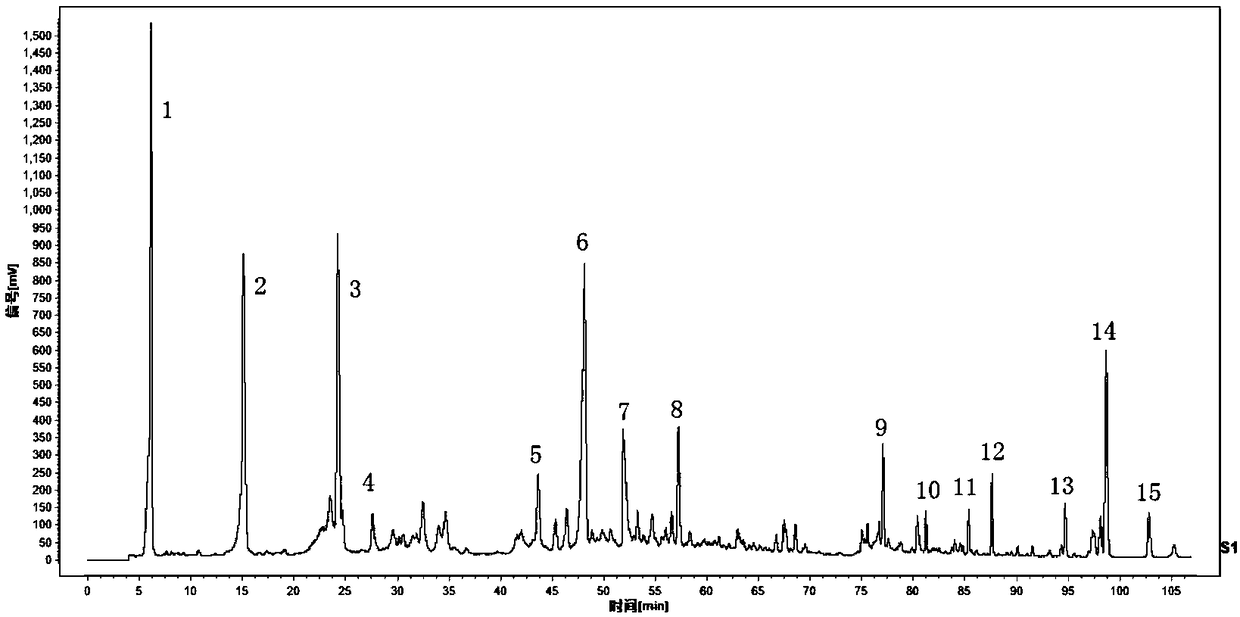
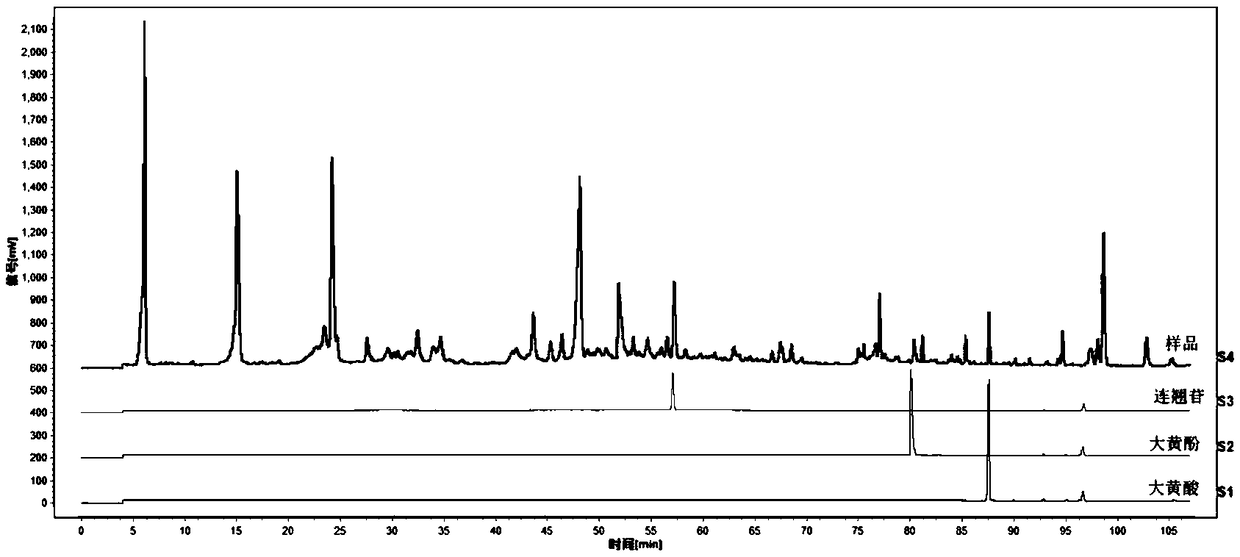
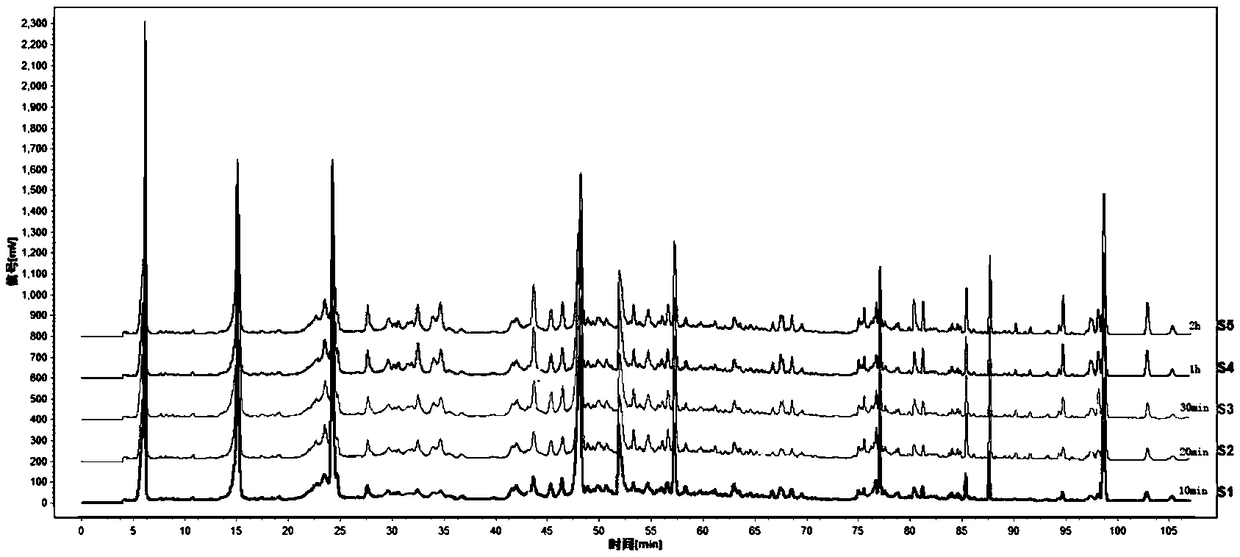
Determination method for HPLC-ELSD fingerprint spectrum of Lotrimin Sol Lotrimin
A technology of Huangshi Xiangsheng Pills and its determination method, which is applied to the determination of fingerprints of Huangshi Xiangsheng Pills preparations, and in the field of fingerprints of Huangshi Xiangsheng Pills preparations, can solve problems such as evaluation, and achieve the effect of perfect quality control technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Step (1) Preparation of the test solution: take Huangshi Xiangsheng pills, pulverize them, take about 4g, accurately weigh them, put them in a conical flask, add 50ml of 85% methanol, ultrasonicate for 1 hour, let cool, filter, and evaporate to dryness , dissolved in 25ml of water, transferred to a separatory funnel, extracted 3 times with ethyl acetate, 25ml each time, combined the ethyl acetate extracts, evaporated to dryness, dissolved the residue with methanol, transferred to a 5ml volumetric flask, and diluted with methanol to scale, shake well.
[0103] Step (2) The concentrations of the reference substance solutions were prepared as follows: the concentration of the forsythin reference substance was 0.3125 mg / ml, the concentration of the chrysophanol reference substance was 0.4981 mg / ml, and the concentration of the rhein reference substance was 0.5306 mg / ml.
[0104] Step (3) need testing solution, reference substance solution is injected into chromatograph, obt...
Embodiment 2
[0114] For the fingerprint determination of batch 170801 of Huangshi Xiangsheng Pills, the specific steps are as follows:
[0115] Step (1) Preparation of the test solution: take Huangshi Xiangsheng pills, pulverize them, take about 4g, accurately weigh them, put them in a conical flask, add 50ml of 85% methanol, ultrasonicate for 1 hour, let cool, filter, and evaporate to dryness , dissolved in 25ml of water, transferred to a separatory funnel, extracted 3 times with ethyl acetate, 25ml each time, combined the ethyl acetate extracts, evaporated to dryness, dissolved the residue with methanol, transferred to a 5ml volumetric flask, and diluted with methanol to scale, shake well.
[0116] Step (2) The concentrations of the reference substance solutions were prepared as follows: the concentration of the forsythin reference substance was 0.3125 mg / ml, the concentration of the chrysophanol reference substance was 0.4981 mg / ml, and the concentration of the rhein reference substance...
Embodiment 3
[0127] For the fingerprint determination of batch 171003 of Huangshi Xiangsheng Pills, the specific steps are as follows:
[0128] Step (1) Preparation of the test solution: take Huangshi Xiangsheng pills, pulverize them, take about 4g, accurately weigh them, put them in a conical flask, add 50ml of 85% methanol, ultrasonicate for 1 hour, let cool, filter, and evaporate to dryness , dissolved in 25ml of water, transferred to a separatory funnel, extracted 3 times with ethyl acetate, 25ml each time, combined the ethyl acetate extracts, evaporated to dryness, dissolved the residue with methanol, transferred to a 5ml volumetric flask, and diluted with methanol to scale, shake well.
[0129] Step (2) The concentrations of the reference substance solutions were prepared as follows: the concentration of the forsythin reference substance was 0.3125 mg / ml, the concentration of the chrysophanol reference substance was 0.4981 mg / ml, and the concentration of the rhein reference substance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com