Method for controlling quality of radix scutellariae medicinal materials
A quality control method and technology of medicinal materials, applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, plant raw materials, etc., can solve problems such as easy formation of peaks, narrow distribution range, unfavorable samples, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
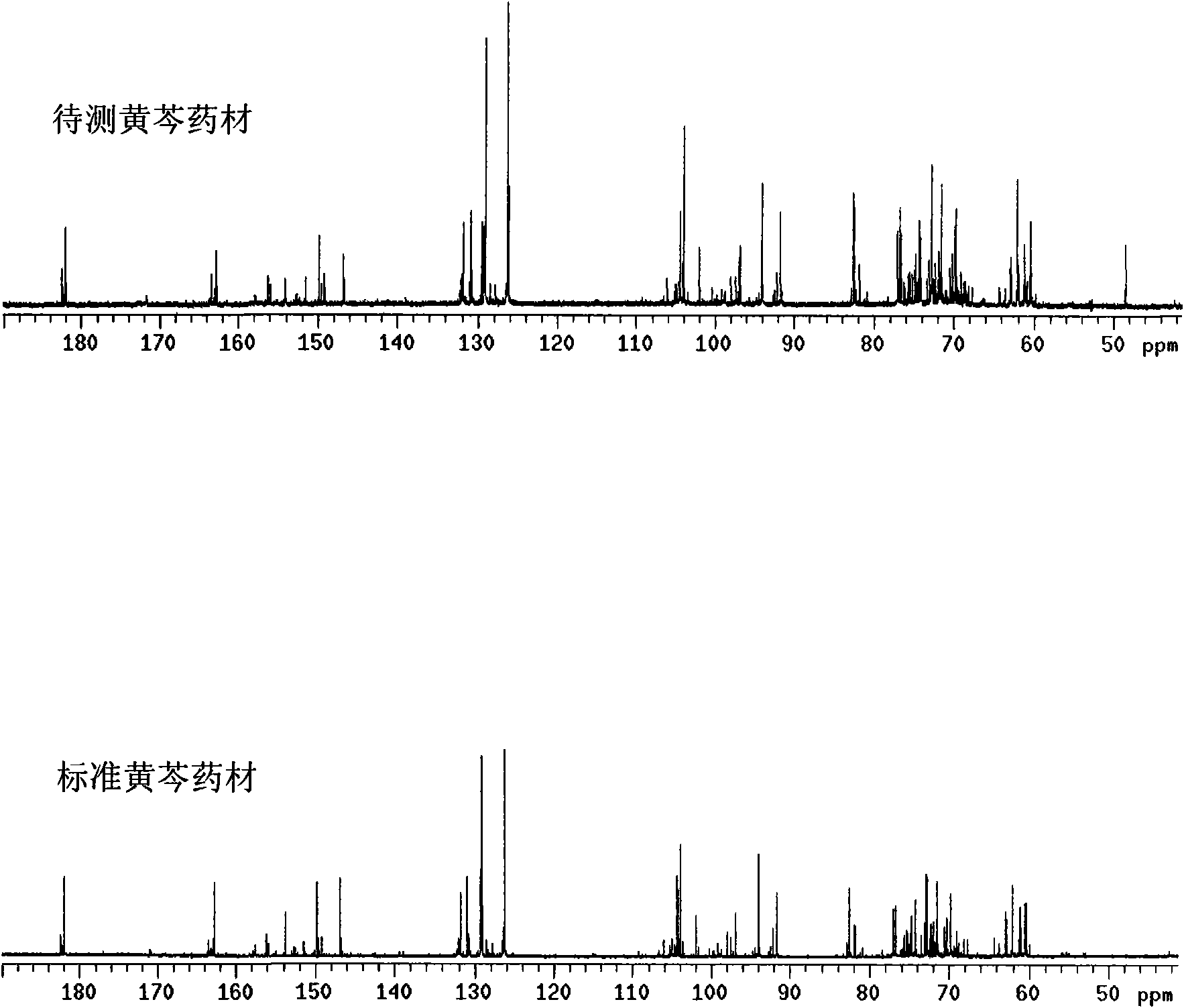
[0023] Embodiment 1: a kind of method that Scutellaria baicalensis medical material fingerprint is established, it is to take the following steps:
[0024] (1) Establishment of standard Scutellaria baicalensis fingerprint
[0025] Preparation of standard Scutellaria baicalensis characteristic extract:
[0026] Take 5 g of standard Scutellaria baicalensis, add 50 mL of 95% ethanol, heat and reflux twice on a water bath, 1 hour each time, filter the extract, volatilize the solvent, and leave the residue for later use.
[0027] Determination of fingerprints of standard Scutellaria baicalensis:
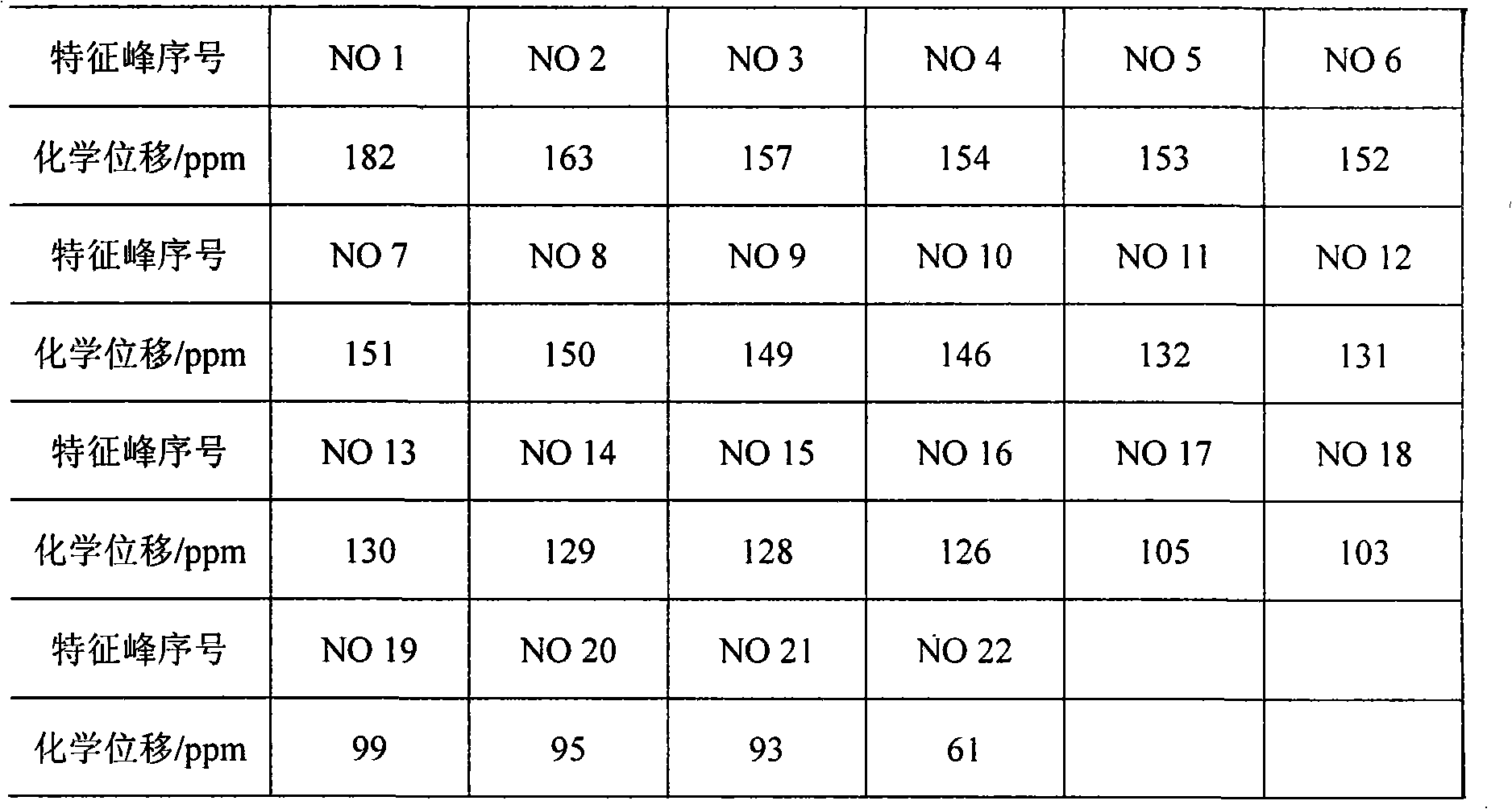
[0028] Accurately weigh 10.0 mg of the characteristic extract dried at 60°C for 4 hours in a nuclear magnetic test tube, and accurately measure 0.5mL DMSO-d 6 Put it in a nuclear magnetic test tube, shake well, and use it for testing. Measuring conditions: Measuring temperature 25°C, scanning times 8000 times, DMSO-d 6 As the solvent, the δ39.49 residual solvent peak was used as the i...
Embodiment 2
[0031] Example 2: (1) Preparation of characteristic extract of standard Scutellaria baicalensis medicinal material sample: Take 5 g of standard Scutellaria baicalensis medicinal material sample, add 50 mL of 95% ethanol, heat and reflux twice on a water bath, each time for 1 hour, filter the extract, and volatilize the solvent , the residue is set aside.
[0032](2) Preparation of characteristic extracts of standard Scutellaria baicalensis medicinal materials: Take 5 g of the test sample, add 50 mL of 95% ethanol, heat and reflux on a water bath twice for 1 hour each time, filter the extract, volatilize the solvent, and leave the residue for later use.
[0033] (3) Determination method: Accurately weigh 20.0 mg of various characteristic extracts dried at 60°C for 4 hours in a nuclear magnetic test tube, and accurately measure 0.5mL DMSO-d 6 Put it in a nuclear magnetic test tube, shake well, and use it for testing. Measuring conditions: Measuring temperature 25°C, scanning ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com