Flavone-O-methyltransferase, and application thereof in synthesis of wogonin, isowogonin and moslosooflavone
A technology of methyltransferase and flavonoids of thalassemia, which is applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Obtaining of Encoding Nucleotide and Construction of Vector
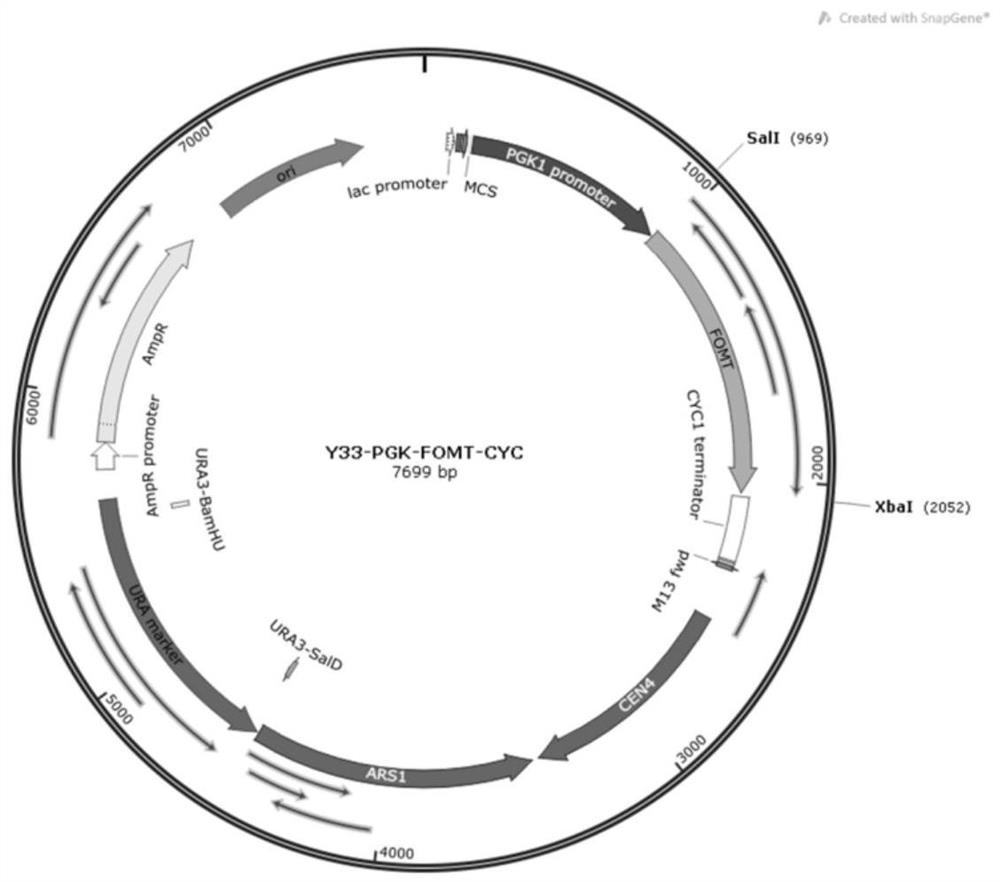
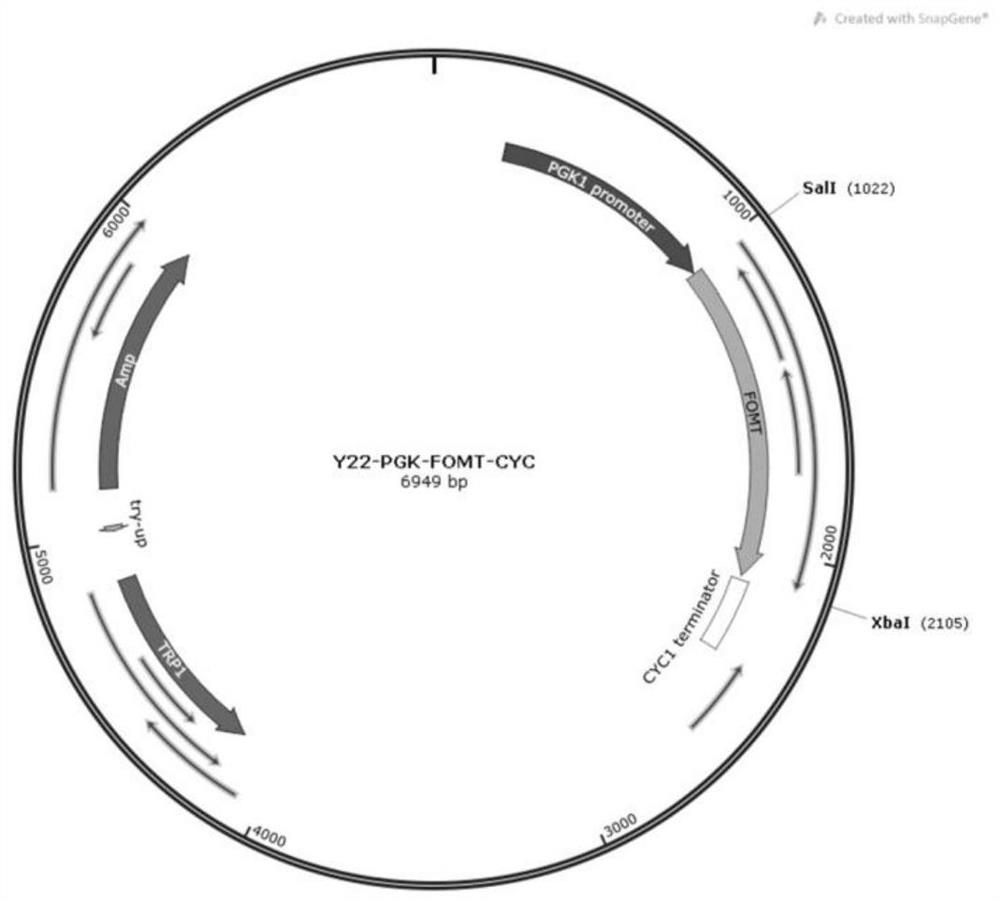
[0052] According to the nucleotide sequence information provided by the present invention (SEQ ID No.8, SEQ ID No.9, SEQ ID No.10, SEQ ID No.11, SEQ ID No.12, SEQ ID No.13, SEQ ID No .14), performing gene synthesis. Using the shuttle plasmid YCPlac33-PE as the carrier, the exogenous sequence was inserted between the restriction sites SalI and XbaI, and the recombinant vector was constructed using the Gibson assembly method ( figure 1 ). In addition, for SEQ ID No.14, the shuttle plasmid YCPlac22-PE was also used as a carrier, and the foreign sequence was inserted between the restriction site SalI and XbaI, and the recombinant vector was constructed using the Gibson assembly method ( figure 2 ).
Embodiment 2
[0053] Example 2 Construction of Wogonin-producing Saccharomyces cerevisiae Host Bacteria
[0054] 2.1 Production of wogonin using nor-wogonin as substrate
[0055] Utilize conventional lithium acetate transformation method to comprise the recombination of nucleotide sequence SEQ ID No.8 or SEQ ID No.9 or SEQ ID No.10 or SEQ ID No.11 or SEQ ID No.12 described in embodiment 1 The vector was transformed into Saccharomyces cerevisiae host strain W303-1B, and the clones that could grow on uracil-deficient complete (CM) medium were picked, and wogonin was fermented with 1mM norwogonin as the substrate .
[0056] 2.2 Production of wogonin with chrysin as substrate
[0057] The recombinant vector comprising the nucleotide sequence SEQ ID No.12 described in Example 1 was transformed into a Saccharomyces cerevisiae host containing flavone-8-hydroxylase (F8H) and P450 reductase (CPR) using a conventional lithium acetate transformation method Bacteria, pick the clones that can grow on...
Embodiment 3
[0058] Example 3 Construction of Saccharomyces cerevisiae host bacteria producing iso-wogonin
[0059] 3.1 Production of iso-wogonin using nor-wogonin as substrate
[0060] The recombinant vector comprising nucleotide sequence SEQ ID No.13 or SEQ ID No.14 described in Example 1 is transformed into Saccharomyces cerevisiae host bacterium W303-1B by conventional lithium acetate transformation method, and picking can be carried out at uracil The clones grown on the deficient CM medium were fermented with norwogonin at a concentration of 1 mM to produce isowogonin.
[0061] 3.2 Production of isowogonin with chrysin as substrate
[0062] The recombinant vector comprising the nucleotide sequence SEQ ID No.14 described in Example 1 was transformed into a Saccharomyces cerevisiae host containing flavone-8-hydroxylase (F8H) and P450 reductase (CPR) using a conventional lithium acetate transformation method Bacteria, pick the clones that can grow on tryptophan and uracil-deficient CM ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com