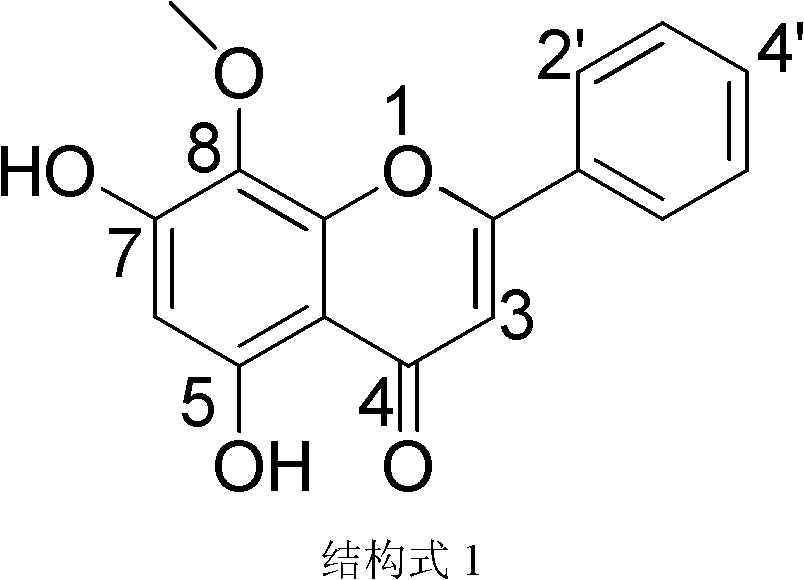
Use of wogonin in preparation of drug for treating chronic kidney disease
A technology of wogonin and chronic kidney disease, which is applied in the field of pharmaceuticals and can solve the problems of no reports of chronic kidney disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
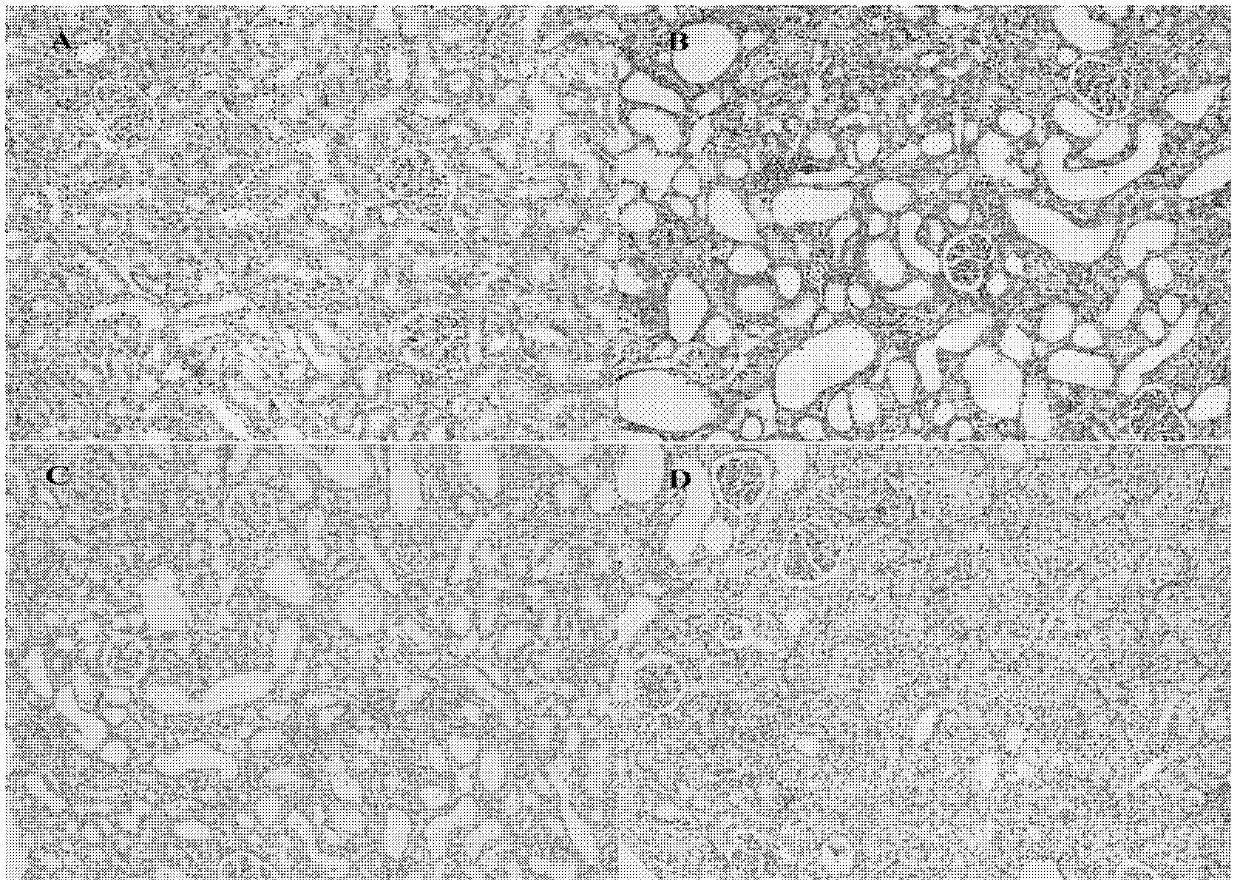
[0027] Example 1 (medicine efficacy test of wogonin on unilateral ureteral ligation (UUO) model rats)
[0028] 1. Purpose of the test: To investigate whether wogonin can improve renal fibrosis in unilateral urinary catheter ligation (UUO) model rats.
[0029] 2. Test method:
[0030] 2.1. Animal grouping
[0031] They were randomly divided into 5 groups: 10 rats in the sham operation group, 10 rats in the model group, 10 rats in the losartan group, 10 rats in the low-dose wogonin group, and 10 rats in the high-dose wogonin group.
[0032] 2.2. Model making
[0033] Except for the sham operation group, the other 4 groups underwent unilateral ureteral ligation. The method was as follows: After the experimental rats were anesthetized with chloral hydrate (400 mg / kg) intraperitoneally, an incision was made in the left abdominal kidney area of the rats, and the left side was separated with ophthalmic forceps. For the ureter, use No. 4 surgical thread to ligate the ureter at th...
Embodiment 2
[0063] Embodiment 2 (medicine efficacy test of wogonin on 5 / 6 nephrectomy model rats)
[0064] 1. Purpose of the test: To investigate whether wogonin can improve renal fibrosis in 5 / 6 nephrectomy model rats.
[0065] 2. Test method:
[0066] 2.1. Animal grouping, model making, drug administration and material collection
[0067] Rats ate standard food, drank water freely, and were adaptively fed for 1 week, and were randomly divided into 5 groups: 10 rats in the sham operation (Sham) group, 10 rats in the model group (5 / 6 nephrectomy), and 10 rats in the losartan group (20mg / kg) 10 rats, 10 rats in the wogonin low-dose (10 mg / kg) group, and 10 rats in the wogonin high-dose group (20 mg / kg) group.
[0068] Except for the sham operation group, the rest were used as the modeling group. Rats in the model group were anesthetized by intraperitoneal injection, and the rats were placed prone to expose the left kidney area. After skin preparation, an oblique outward incision was m...
Embodiment 3
[0088] Example 3 (medicine efficacy test of wogonin prodrug on unilateral ureteral ligation (UUO) model rats)
[0089] 1. Purpose of the test: To investigate whether wogonin prodrug can improve renal fibrosis in 5 / 6 nephrectomy model rats.
[0090] 2. Test method: Taking wogonin ethyl ester (the hydrogen on the 7-hydroxyl group of wogonin is esterified) as an example, the method is the same as in Example 1.
[0091] 3. Test results: Wogonin prodrug can increase the weight of model rats, significantly inhibit the increase of serum creatinine and blood urea nitrogen content in chronic kidney disease animals, increase creatinine clearance rate, improve renal function; significantly reduce renal tissue hydroxyproline acid content.
[0092] 4. Experimental conclusion: Wogonin prodrug can improve renal fibrosis in unilateral ureteral ligation (UUO) model rats.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com