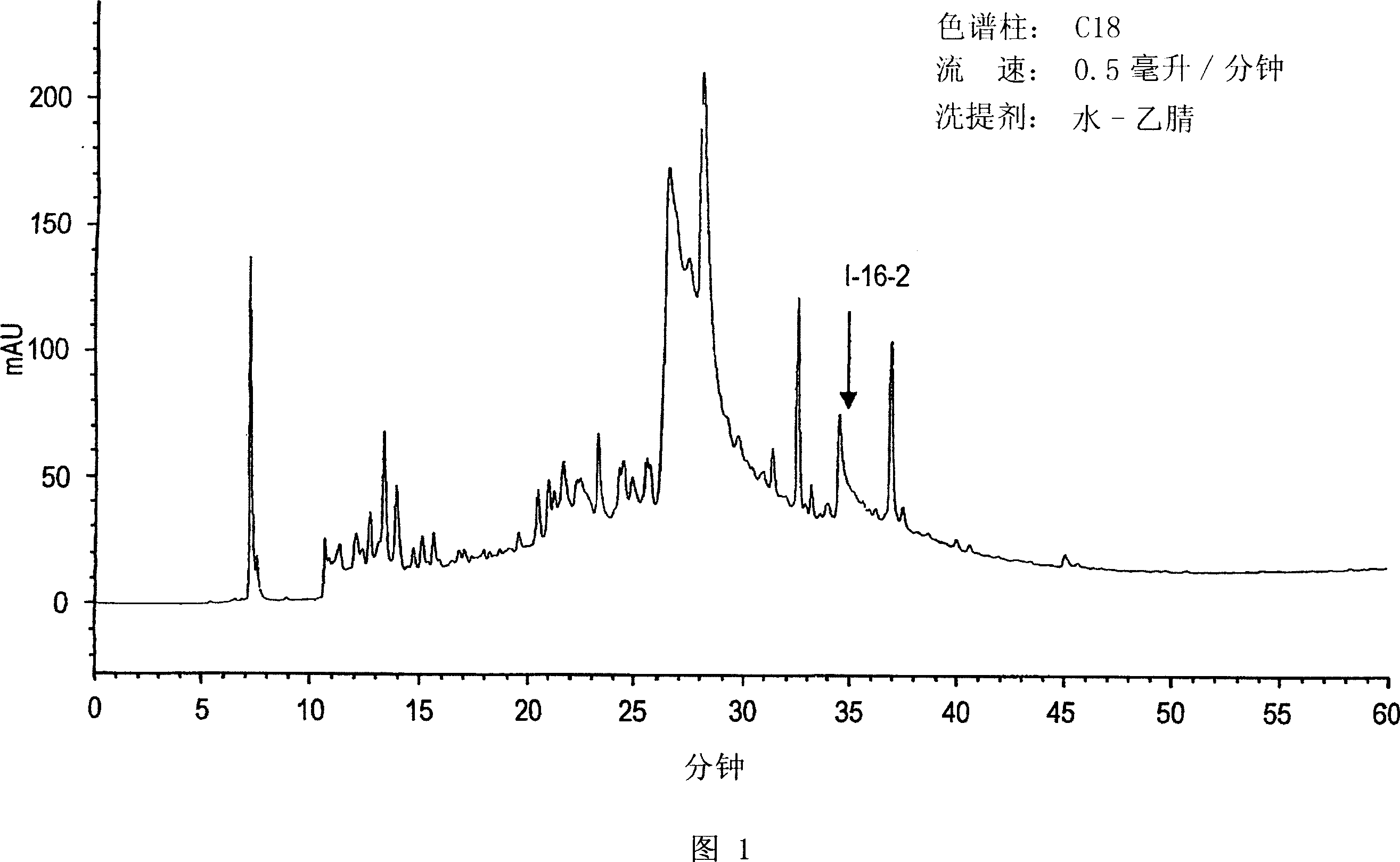
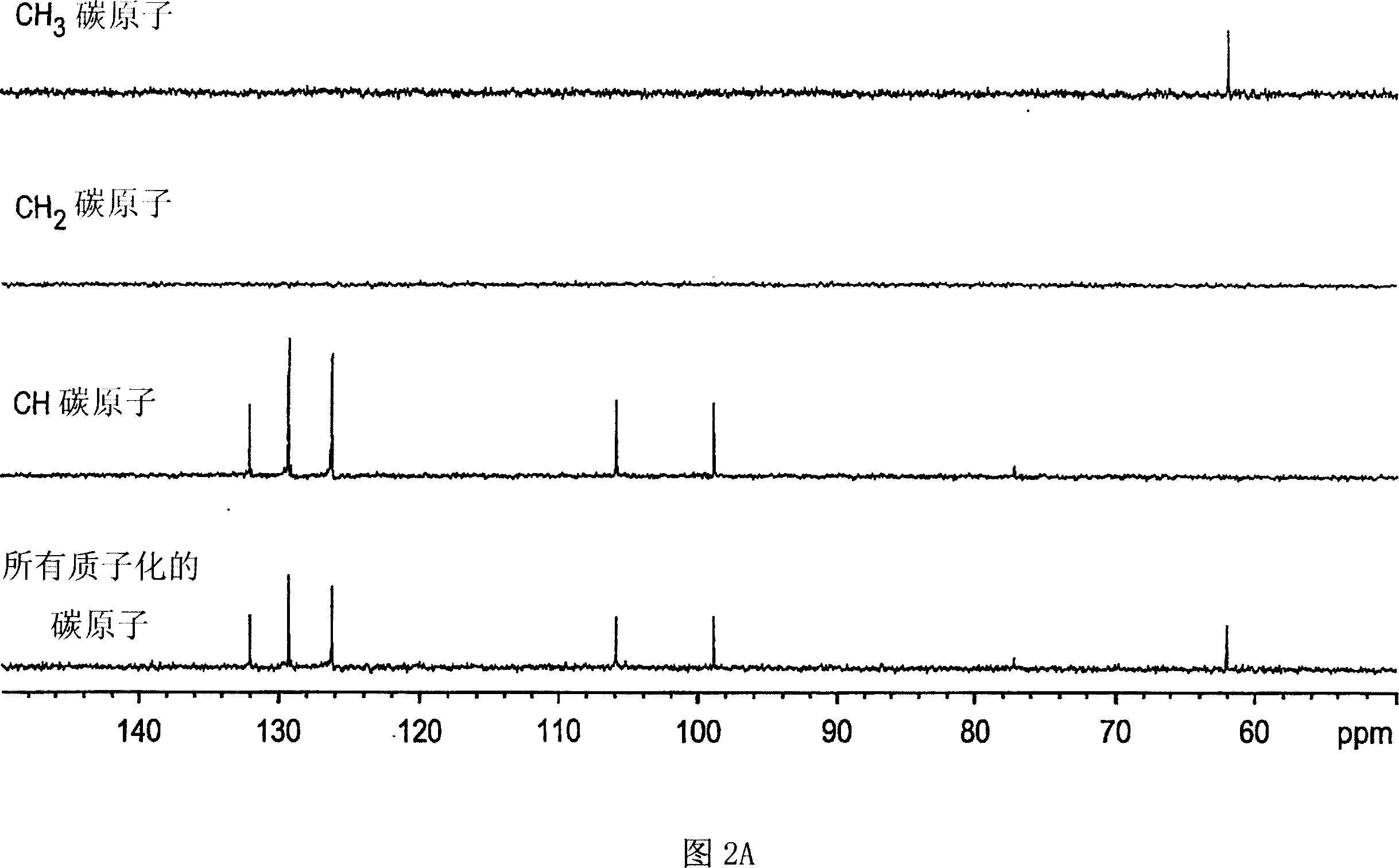
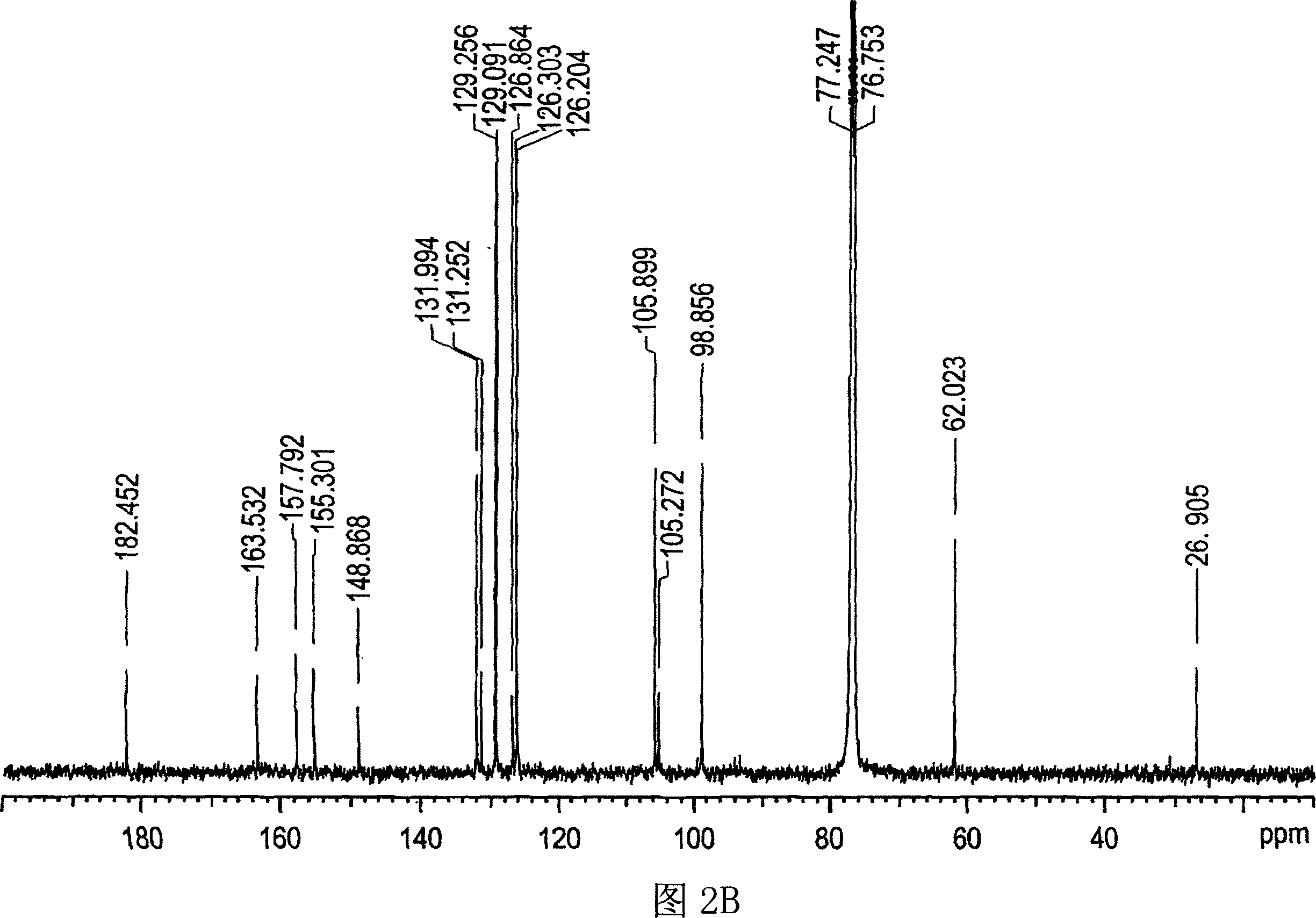
Botanical extract compositions and methods of use
A technology of composition and extract, which is applied in the field of treating cancer and estrogen-related disorders, and can solve problems such as no effect on advanced prostate cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] This example shows the activity of wogonin and isoliquiritigenin in inhibiting the growth of hormone-sensitive prostate cancer cell line LNCAP cells.
[0106]The MTT assay (MTT is 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was used to count viable cell numbers. Detection reagents were purchased from Boehringer Mannheim (Roche Diagnosis Corp, Indianapolis, Indiana). In this assay, the tetrazolium dye MTT is separated into formazan by metabolically active cells and exhibits strong absorption at 550-618 nm. Protocols for cell survival experiments were provided by the manufacturers of the reagents and were further modified by our laboratory as follows.
[0107] Prostate cancer cells or breast cancer cells were seeded in 96-well microtiter plates at a cell density of 3×103 cells (MCF-7) or 6×103 cells (LNCAP or DU145), respectively, and the volume of culture medium was 100 μl . After 24 hours of incubation, 20 microliters of solutions containing differe...
Embodiment 2
[0118] This example shows the activity of isoliquiritigenin in inhibiting the growth of the breast cancer cell line MCF-7.
[0119] The efficacy of isoliquiritigenin on MCF-7 cells was evaluated using the same protocol as described in the Examples. MCF-7 cells are breast cancer cells expressing estrogen receptors. Therefore, it is a good model for studying the effect of antineoplastic drugs on estrogen receptor positive breast cancer.
[0120] Figure 9 shows the MTT experimental curve of isoliquiritigenin on MCF-7 cells. The data showed that isoliquiritigenin inhibited the growth of MCF-7 cells, and a dose-dependent curve was observed. Its ED50 value is shown in column 3 of Table 1.
Embodiment 3
[0122] This example shows the regulatory effect of wogonin and isoliquiritigenin on the cell cycle of LNCaP. LNCAP cells have a hormone-dependent cell cycle.
[0123] Preparation of cell cycle assay samples: cells cultured in 12.5cm2 size flasks (2-4×10 6 cells) were exposed to two concentrations of wogonin and isoliquiritigenin for 24-48 hours before collection. Cells were washed with PBS buffer and fixed in ice-cold 70% ethanol. The fixed cells were extracted, redissolved in PBS solution, and then washed with 1.0 mg / ml DAPI (4,6-diamidino-2-phenylindole, purchased from Eastman Kodak, Rochester, NY (Issey, Rochester, New York). Terman Kodak) stained, and dissolved in as previously described by Halicka et al. by Extracts of the Chinese Herbal Preparation of PC SPES ", INTERNATIONAL J.OF ONCOLOGY (1997), 11:437-448) pH 6.8 containing 100mM NaCl, 2mM MgCl2, and 10mM of 0.1% Triton X-100 (Sigma) piperazine-N,N-bis-2-ethanesulfonic acid buffer.
[0124] Intracellular DNA cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com