Inhibitor or promoter of uridinediphosphate glucuronosyltransferase 2B (UGT2B)
A technology of uridine diphosphate and glucose, which is applied in the field of UGT2B accelerators and effective UGT2B inhibitors, and can solve the problem of low clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1. In Vitro Experiments of UGT 2B Inhibitors
[0070] Materials and Methods:
[0071] 1. Preparation of UGT2B inhibitors
[0072] In the following experiments, the present invention uses 27 kinds of traditional Chinese medicine medicines and 10 kinds of excipients as UGT2B inhibitors, wherein the Chinese medicine medicines are all commercial pure compounds, purchased from Sigma Chemical Co., Nacalai Tesque (Kyoto, Japan) and INDOFINE Chemical Co., Inc. (Somerville, New Jersey). The types, names, crude drug sources and chemical formulas of these traditional Chinese medicines are listed in Table 1 below. These traditional Chinese medicines were formulated with ethanol to concentrations of 1, 10, and 100 μM, respectively, for subsequent experiments.
[0073] In addition, the excipients are all commercial pure compounds, namely PEG (Polyethyleneglycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ 58 BRIJ 76. Pluronic F68, Pluronic F127....
Embodiment 2
[0113] Example 2 In Vitro Experiment of UGT 2B Accelerators
[0114] This example is carried out with the same procedure as that described in Example 1, but 40 kinds of traditional Chinese medicine medicines adopted in the following list 5 are used as UGT2B accelerators, and these Chinese medicine medicines are all commercialized pure Compounds were purchased from Sigma Chemical Co., Nacalai Tesque (Kyoto, Japan) and INDOFINE Chemical Co., Inc. (Somerville, New Jersey), respectively. The types, names, crude drug sources and chemical formulas of these traditional Chinese medicines are listed in Table 5 below.
[0115] Table 5 The types, names, sources and chemical formulas of UGT2B accelerators
[0116]
[0117]
[0118]
[0119]
[0120]
[0121] result:
[0122] The results of the above experiments are shown in Table 6. Orthoguaic acid has the best promoting effect on liver microsomal metabolism of nalbuphine, which can reach -188.09 (±16.566)% i...
Embodiment 3
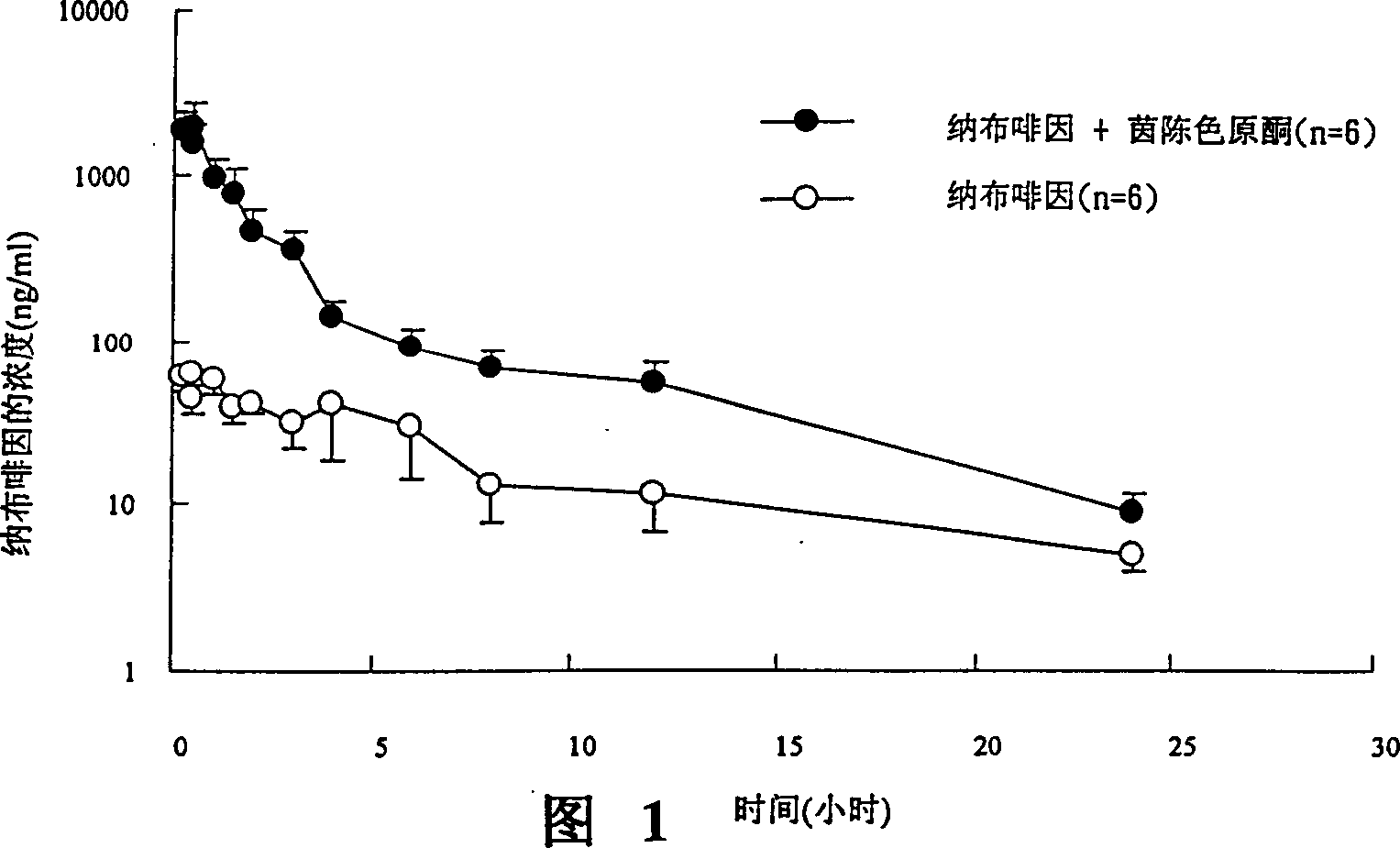
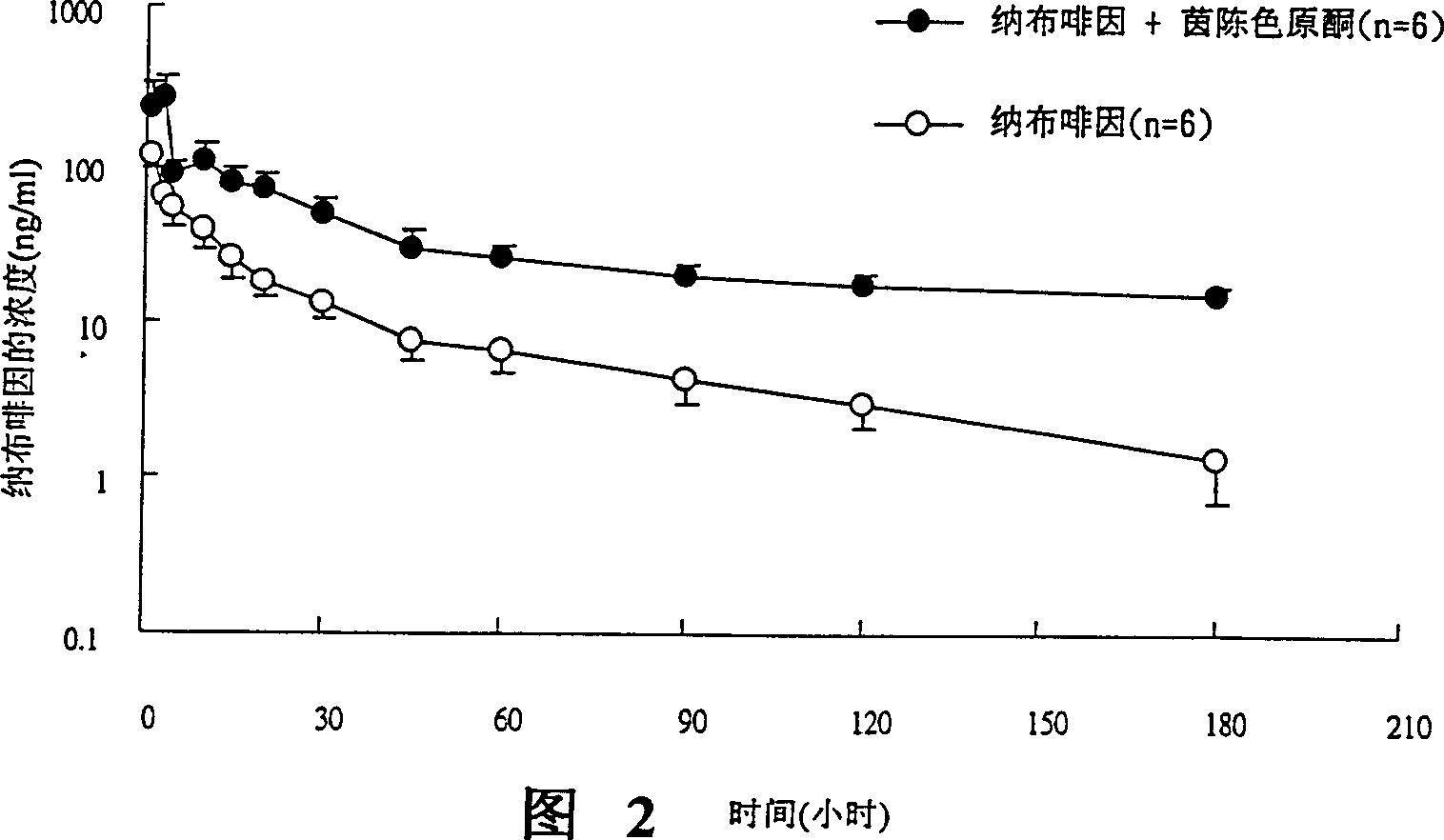
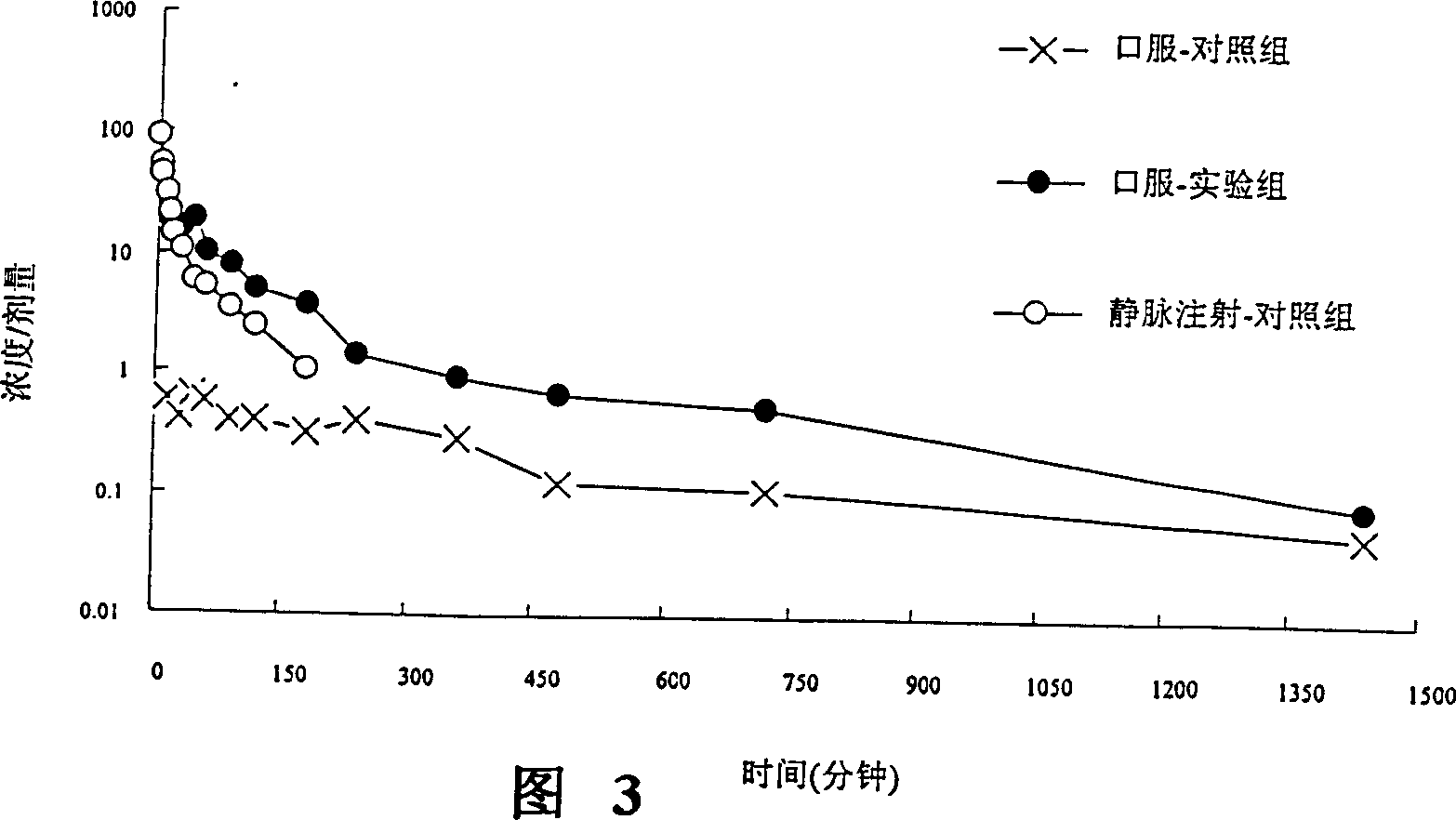
[0124] Example 3 Effect of UGT 2B Inhibitors on Oral Concentration of Nalbuphine
[0125] Materials and Methods:
[0126] 1. Experimental animals
[0127]The source of animals is the Sprague-Dawley strain of male rats used in experiments, mainly healthy rats with a body weight of 500-600 g, purchased from the National Laboratory Animal Breeding and Research Center, Taiwan. After purchase, the animals were given a one-week adaptation period, and were reared at a fixed temperature (25±1° C.), humidity and photoperiod (12 hours of light per day). Fast for about 12-16 hours before the experiment. The experimental method is to evaluate drug absorption in an oral way.
[0128] 2. Preparation of UGT2B inhibitor and nalbuphine
[0129] The standard solution of nalbuphine is prepared in water, and the inhibitor is prepared in alcohol.
[0130] 3. Experimental method:
[0131] i. After intraperitoneal injection (I.P.) of 3-5mg / 100g body weight of pentobarbital (pentobarbital), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com