New application of chemical component of eucommia bark used as plant estrogen
A technology of estrogens and uses, applied in the field of new uses of Eucommia ulmoides as phytoestrogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1, the preparation of active ingredient monomer
[0065] Cut 31.5 kg of eucommia medicinal material (purchased from Henan Xinyang Medicinal Material Company) into small pieces, add 315 liters of 95% ethanol to soak overnight, reflux extraction for 2.5 hours, pour out the extract, pour out the extract, add 315 liters of 60% ethanol to the dregs Ethanol was refluxed for 2 hours, the extracts were combined and filtered, and the ethanol was recovered from the filtrate, followed by extraction with petroleum ether, chloroform, ethyl acetate and n-butanol, and the solids were obtained after recovering the solvents respectively. Results: 153g of petroleum ether partial solids, 384g of chloroform partial solids, 126g of ethyl acetate partial solids, and 520g of n-butanol partial solids.
[0066] After 350 g of Eucommia chloroform part was subjected to silica gel column chromatography, 33-36 fractions were subjected to repeated silica gel column chromatography to obta...
Embodiment 2
[0069] Embodiment 2, the structural confirmation of active ingredient
[0070] 11 compounds obtained in Example 1 (i.e., betulinic acid, genipin, aucubin, pinoresinol diglucoside, syringaresin diglucoside, pinoresinol monoglucoside, syringaresin monoglucoside , wogonin, baicalein A, baicalein, and dihydrochalcone 3-O-β-D-glucoside) for structural confirmation (entrusted Tianjin University Analysis and Testing Center detection, 500MHz NMR). The analysis results showed that the structures of these compounds were consistent with those reported in the literature, as follows.
[0071] Betulinic acid, white needle crystal. 1 H-NMR (C 5 D. 5 N, 500MHz): δ4.90 (1H, s, H-29a), 4.72 (1H, s, H-29b), 3.49 (1H, m, H-3), 1.74 (3H, s, H-30) , 1.18(3H, s, H-26), 1.02(3H, s, H-27), 1.01(3H, s, H-23), 0.96(3H, s, H-25), 0.77(3H, s , H-24).
[0072] Betulin, white powder. 1 H-NMR (CDCl 3 , 500MHz): δ4.68, 4.58 (2H, dd, J=11.5, 4.5Hz, H-29), 3.80, 3.33 (2H, dd, J=11.5, 4.5Hz, H-28), 2.3...
Embodiment 3
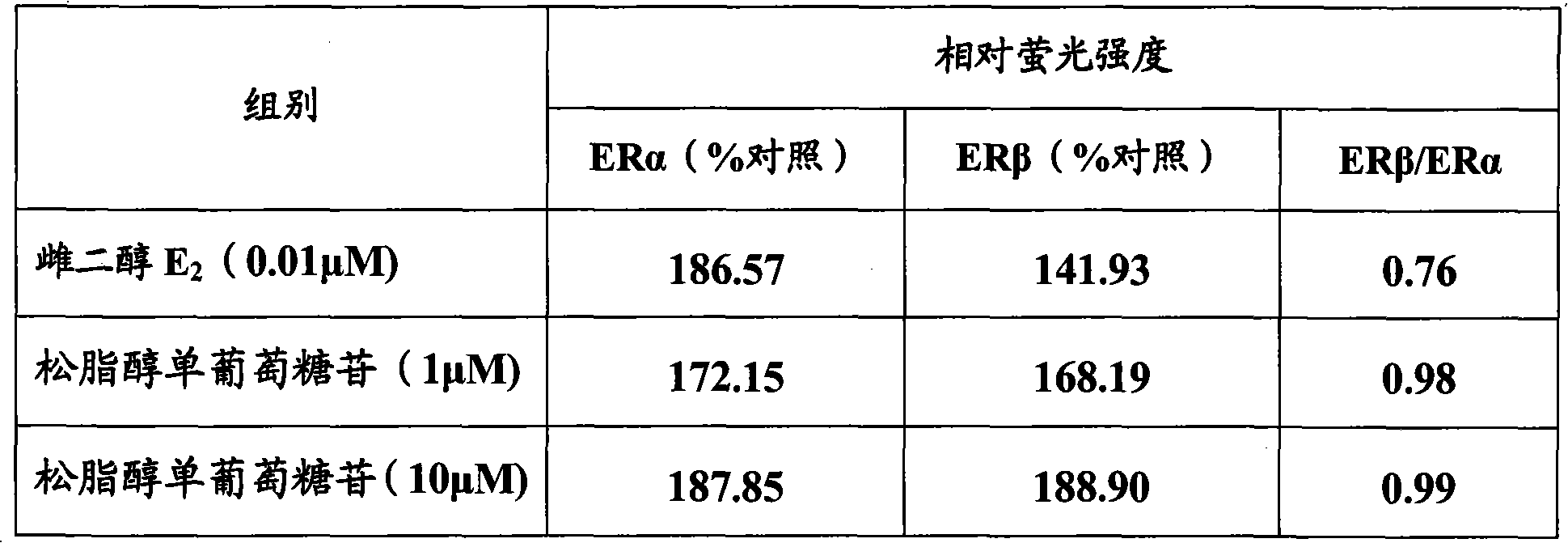
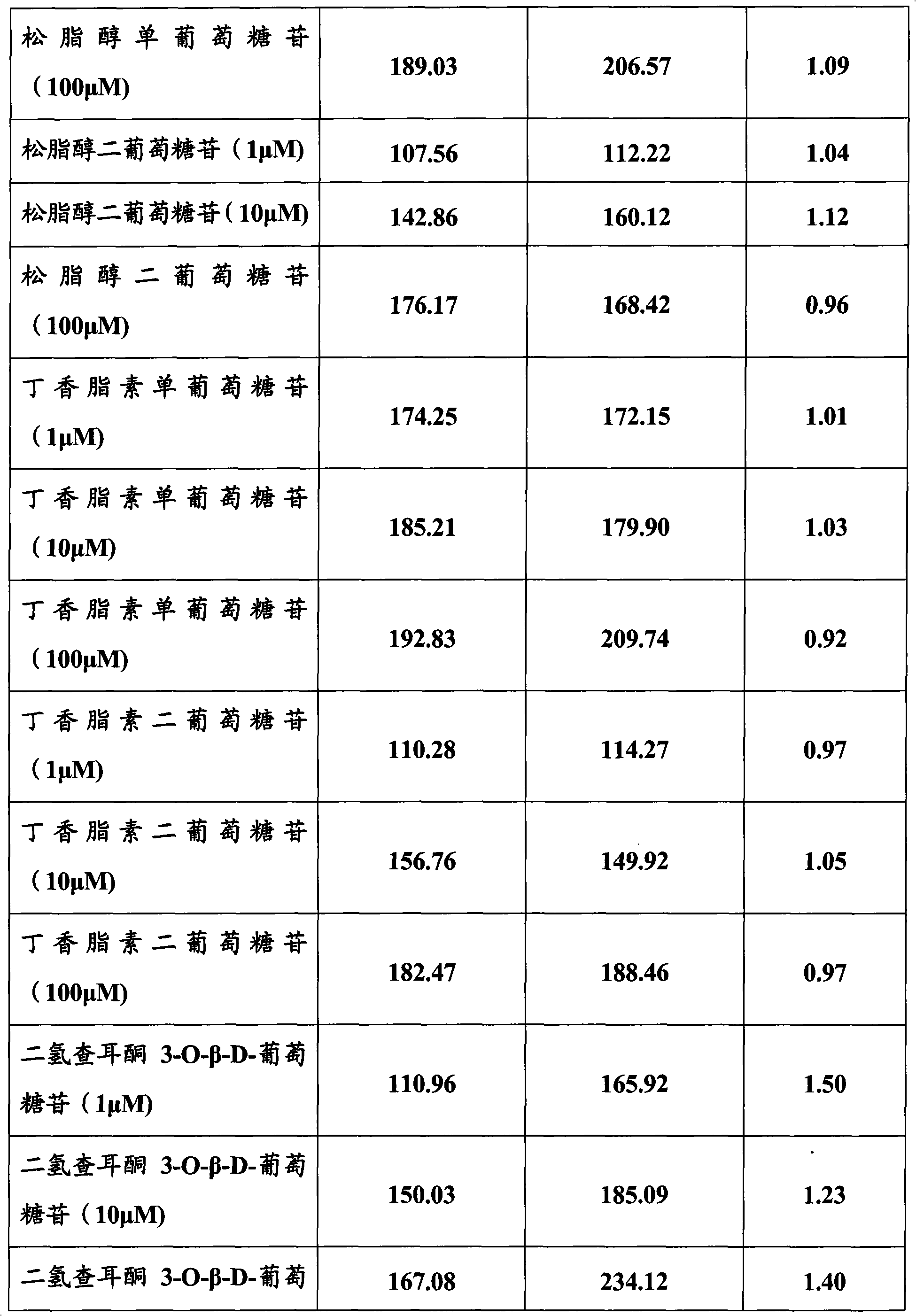
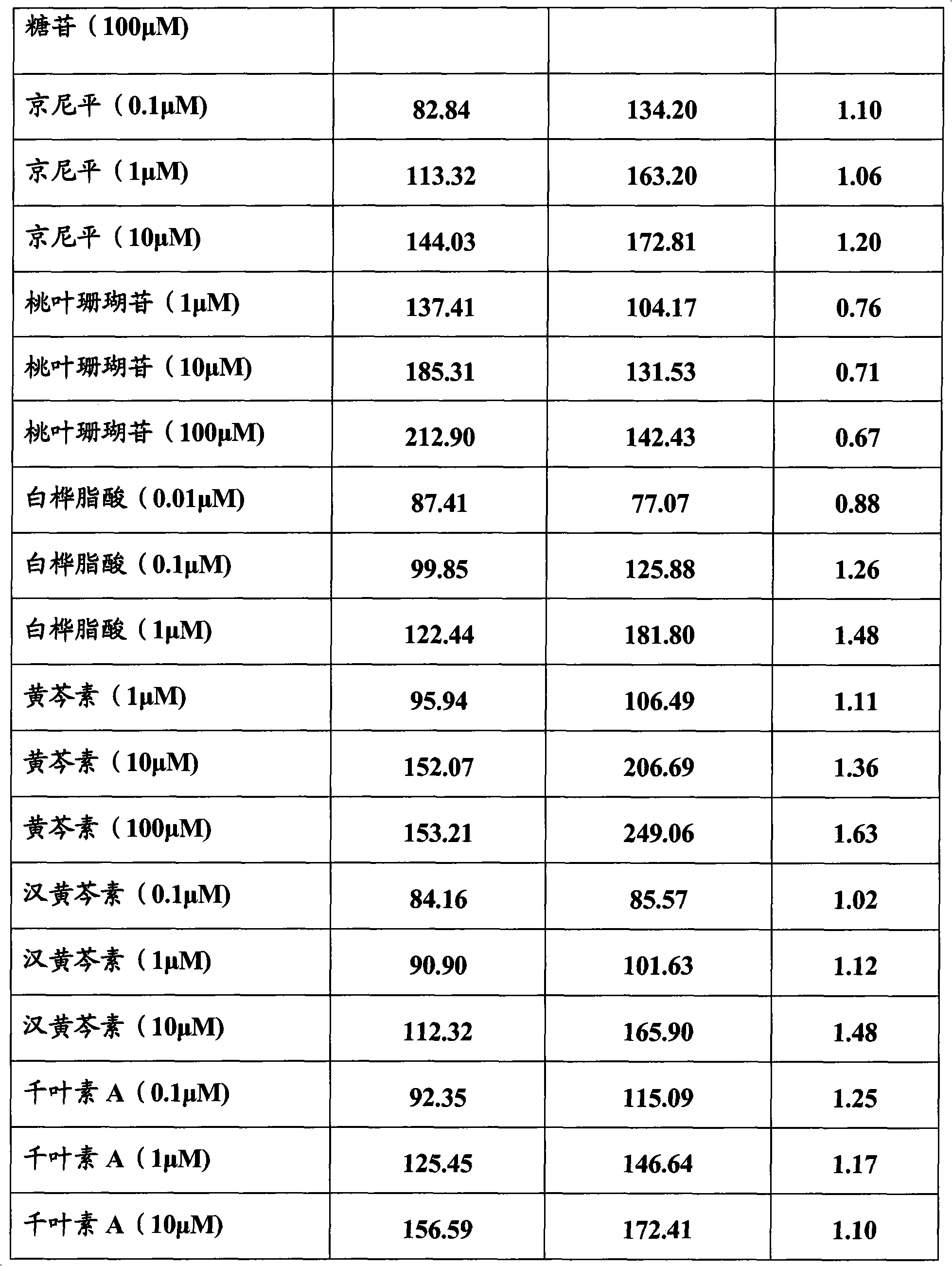
[0083] Embodiment 3, the activation effect of the compound of the present invention on estrogen receptor
[0084]Cell transfection method was used to observe betulinic acid, genipin, aucubin, pinoresinol diglucoside, syringaresin diglucoside, pinoresinol monoglucoside, syringaresin monoglucoside, wogonin, Selective activation of estrogen receptors by chilesin A, baicalein, and dihydrochalcone 3-O-β-D-glucoside. In fact, estrogen exhibits its biological activity by regulating the transcription of estrogen-responsive genes.
[0085] ER with reporter gene α or ER β The NA fragments were respectively transfected into Hela cells without ER expression, and after the test substance was added to the cell culture medium, it was observed whether the test substance could pass through the ER by detecting the transcriptional activation of the reporter gene. α or ER β exert estrogen-like effects.
[0086] Betulinic acid, genipin, aucubin, pinoresinol diglucoside, syringaresin digluco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com