Method for establishing fingerprint spectrum of liver-enhancing medicine
A method and a technology for establishing fingerprints, which are applied in the field of establishing high-performance liquid chromatography fingerprints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1 adopts HPLC method to establish the fingerprint of Qianggan Capsule contents
[0097] Preparation of reference substance solution: Accurately weigh 2.54 mg of paeoniflorin reference substance, put it in a 25ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, and obtain paeoniflorin reference substance solution (0.1016mg / mL).
[0098] Preparation of the test solution: Weigh 1.0 g of the contents of Qianggan Capsules, add 100 ml of 70% methanol aqueous solution, ultrasonically extract for 1 h, let cool, centrifuge for about 10 min, take the supernatant and concentrate to about 10 ml, dissolve with 25 ml of water, Extract with saturated n-butanol 3 times with 30ml, 20ml, and 20ml each time, combine the 3 extracts, concentrate to dryness, dissolve in methanol and transfer to a 10ml volumetric flask, filter with a 0.45μm filter membrane, discard the initial The filtrate is obtained by taking the continued filtrate and set aside. ...
Embodiment 2
[0104] Methodology investigation of embodiment 2 fingerprint collection method
[0105] 1) Precision test
[0106] Using the same operation and conditions as in Example 1, the same test solution was accurately measured, and the sample was injected continuously for 5 times to investigate the consistency of the relative retention time and relative peak area of the chromatographic peaks. Taking paeoniflorin as the reference peak, the relative retention time and relative peak area of each chromatographic peak were calculated.
[0107] The results showed that the relative standard deviation RSD of the relative retention time of each chromatographic peak was lower than 0.5%, and the relative standard deviation RSD of the relative peak area of each chromatographic peak was lower than 3.9%, which met the requirement of fingerprint.
[0108] 2) Stability inspection
[0109] Using the same operation and conditions as in Example 1, prepare the test solution, place it airtight a...
Embodiment 3
[0114] Example 3 Establishment of the Control Fingerprint of Qianggan Capsule Contents
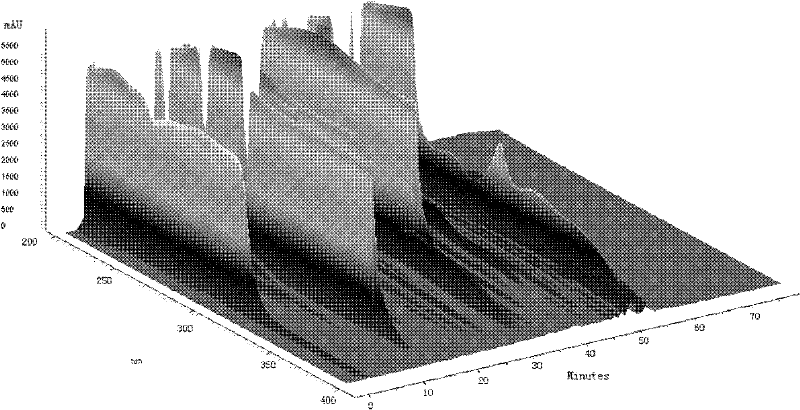
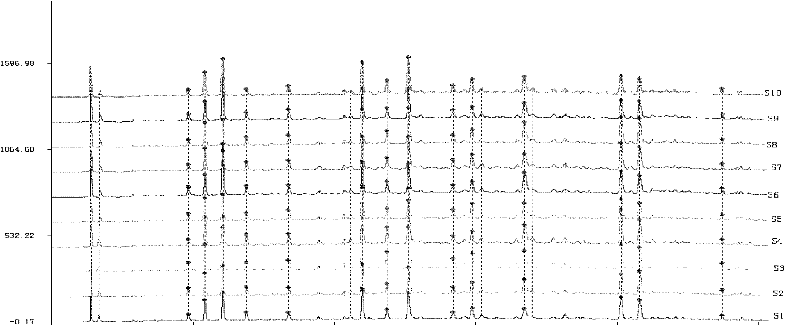
[0115] According to the method described in Example 1, the contents of Qianggan Capsules in batches S1 to S38 were analyzed and determined to obtain fingerprints.
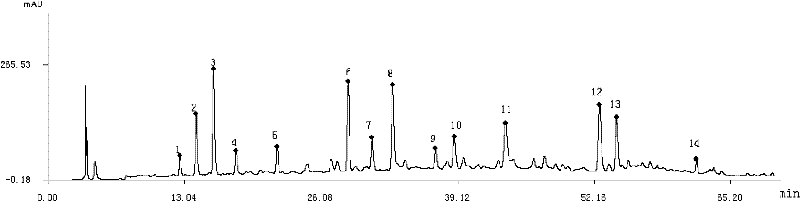
[0116] In the obtained fingerprint spectrum, there are 14 absorption peaks of chemical components in total, among which there are 8 absorption peaks with a single peak area exceeding 5%, and their characteristics are as follows:
[0117] For peak No. 2, the average retention time RT is 14.178min, the RSD is 0.41%, the peak area is 1743.0, and the RSD is 10.15%;
[0118] For peak No. 3, the average retention time RT is 15.812min, the RSD is 0.22%, the peak area is 2869.8, and the RSD is 21.26%;
[0119] For peak No. 6, the average retention time RT is 28.666min, the RSD is 0.07%, the peak area is 2779.5, and the RSD is 7.71%;
[0120] For peak No. 8, the average retention time RT is 32.911min, the RSD is 0.07%, the peak area...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mobile phase a | aaaaa | aaaaa |
| Flow | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com