Paeoniflorin aromatic ester derivative, preparation method and applications thereof
A technology for paeoniflorin and derivatives, which is applied in the field of paeoniflorin derivatives and achieves the effects of high yield, mild technical route conditions and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
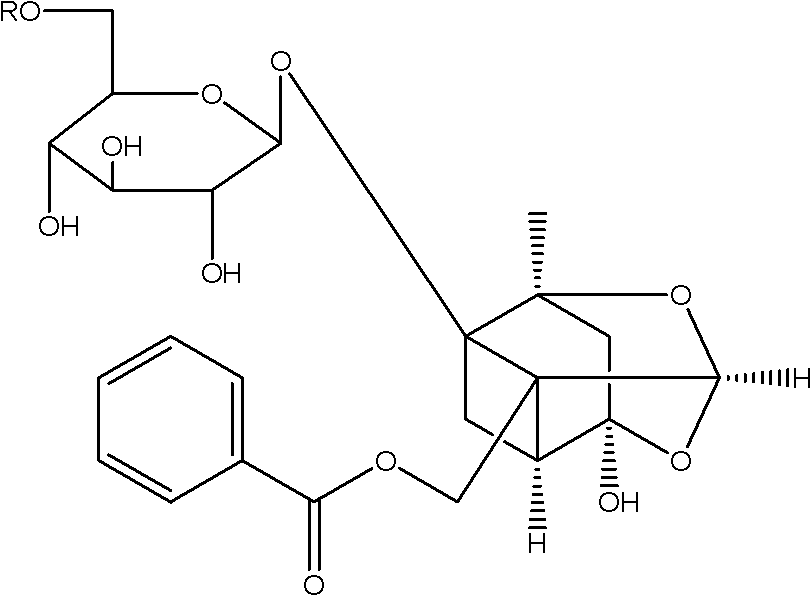
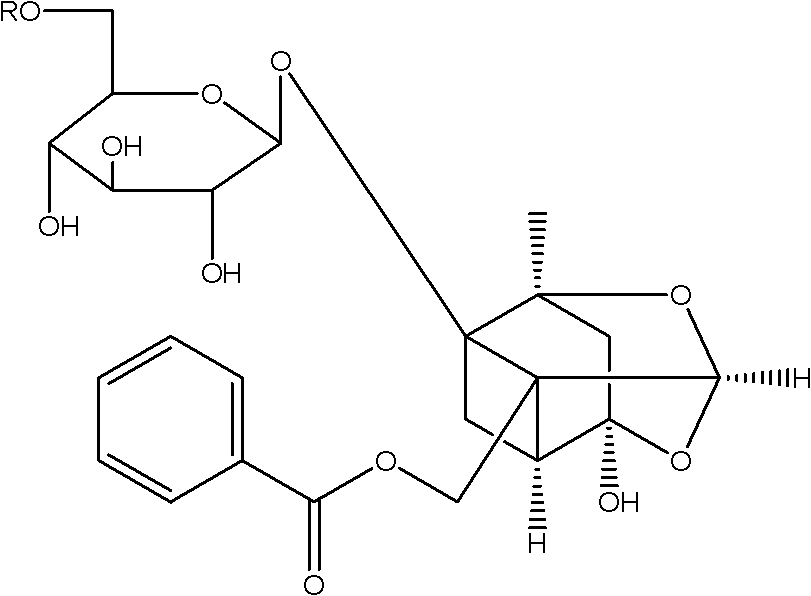
[0034] In a 500ml three-necked flask equipped with an electromagnetic stirrer and a thermometer, 3g of paeoniflorin, 60ml of pyridine, 60ml of chloroform, and 76mg of dimethylaminopyridine were sequentially added. At room temperature (25°C), within 4 hours, slowly add benzoyl chloride solution (604ul benzoyl chloride dissolved in 30ml of chloroform) dropwise to the three-necked flask, after the dropwise addition, continue to stir for 8 hours, the reaction Finish. The reaction solution was washed three times with 120ml (40ml×3) of water, and the organic layer was dried over anhydrous sodium sulfate and filtered. The organic layer was concentrated under reduced pressure and purified by silica gel column chromatography (eluent: trichloromethane:methanol=35:1) to obtain pure benzoylpaeoniflorin as a white crystalline solid with a yield of 45%.
[0035] 1 HNMR (DMSO): δ (ppm):
[0036] 1.34 (3H, s, 10-H), 1.80 (1H, d, J = 12.5Hz, 3α-H), 1.93 (1H, d, J = 10.8Hz, 7α-H), 2.10 (1H, ...
Embodiment 2
[0042] In a 500ml three-neck flask equipped with an electromagnetic stirrer and a thermometer, add 3g of paeoniflorin, 45ml of pyridine, 150ml of chloroform, and 120mg of dipropylaminopyridine in sequence, and at room temperature (25°C) within 4 hours, the benzyl The acid anhydride solution (1.2ml of benzoic anhydride dissolved in 120ml of chloroform) was slowly added dropwise into the three-neck flask, and after the dropwise addition was completed, the stirring was continued for 4 hours, and the reaction was completed. The reaction solution was washed three times with 120ml (40ml×3) of water, and the organic layer was dried over anhydrous sodium sulfate and filtered. The organic layer was concentrated under reduced pressure and purified by silica gel column chromatography (eluent: trichloromethane:methanol=40:1) to obtain pure benzoylpaeoniflorin as a white crystalline solid with a yield of 37%.
[0043] 1 HNMR (DMSO): δ (ppm):
[0044] 1.34 (3H, s, 10-H), 1.80 (1H, d, J = ...
Embodiment 3
[0050] In a 500ml three-necked flask equipped with an electromagnetic stirrer and a thermometer, 3g of paeoniflorin, 90ml of pyridine, and 114mg of dimethylaminopyridine were sequentially added. Slowly add the benzenesulfonyl chloride solution (1.5ml of benzenesulfonyl chloride dissolved in 100ml of methylene chloride) dropwise into the three-necked flask at 35°C within 4 hours. After the dropwise addition, continue stirring for 20 hours to complete the reaction. The reaction solution was washed three times with 120ml (40ml×3) of water, and the organic layer was dried over anhydrous sodium sulfate and filtered. The organic layer was concentrated under reduced pressure and purified by silica gel column chromatography (eluent: chloroform: methanol = 25:1) to obtain pure benzenesulfonyl paeoniflorin as a white crystalline solid with a yield of 41%.
[0051] 1 HNMR (DMSO): δ (ppm):
[0052] 1.14 (3H, s, 10-H), 1.66 (1H, d, J = 12.5Hz, 3α-H), 1.69 (1H, d, J = 10.8Hz, 7α-H), 1.91 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com