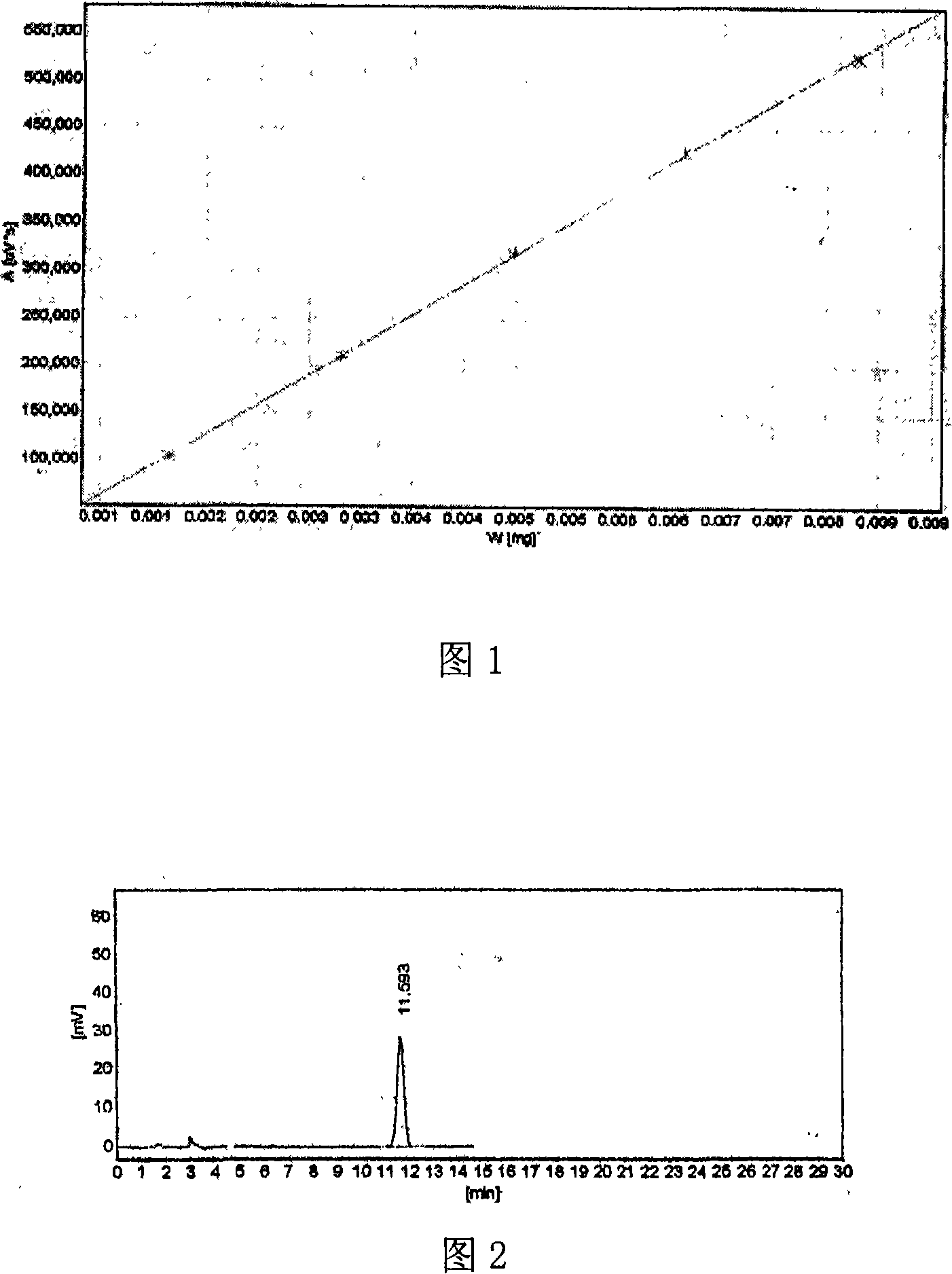
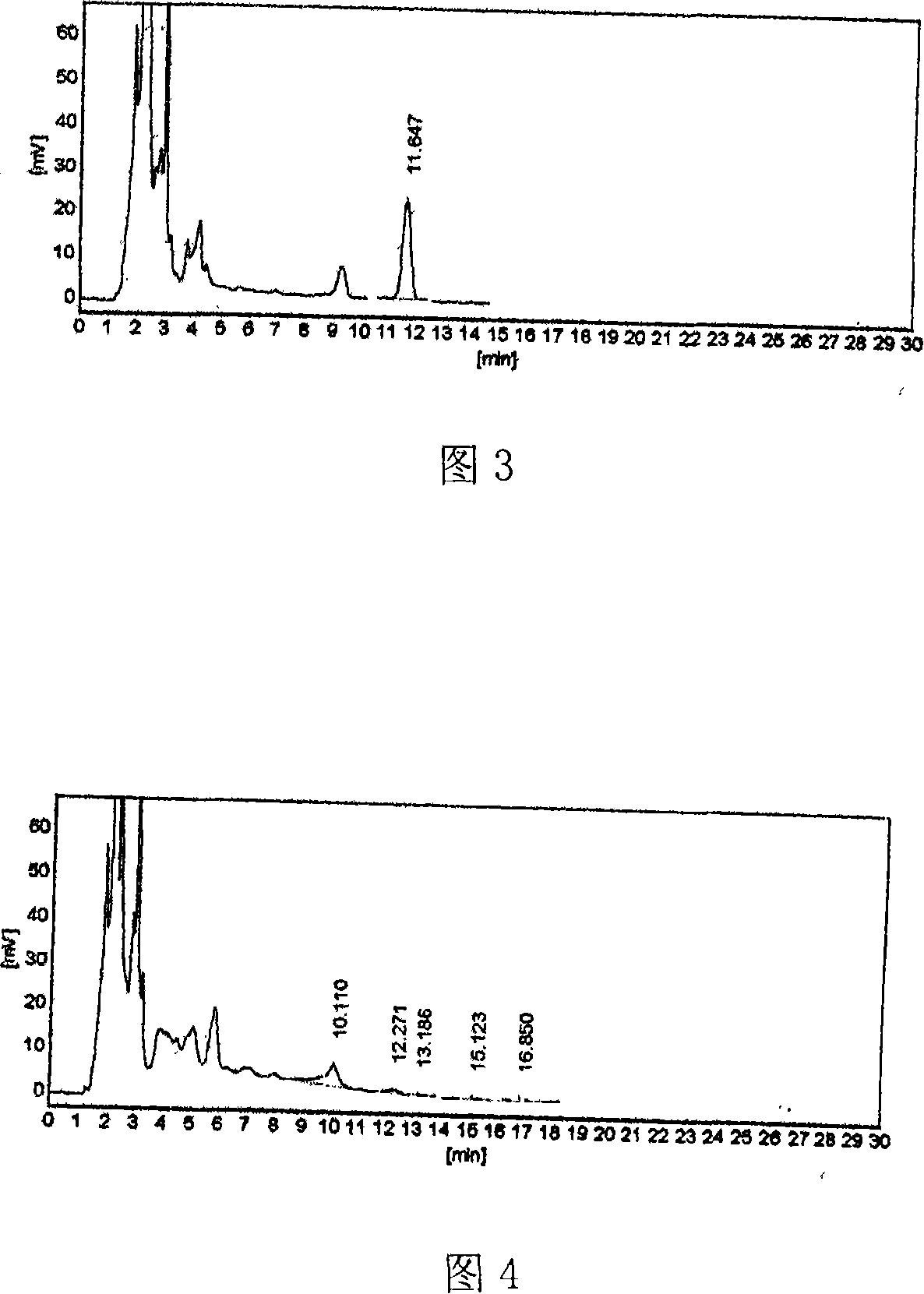
Quality standard of Moluo Dan.and detecting method thereof
A quality control method and content technology, applied in the direction of measuring devices, material inspection products, testing pharmaceutical preparations, etc., can solve the problems of no content measurement indicators, ineffective control of product quality, and few detection indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: the quality control method of Morodan
[0107] Identification method:
[0108] A. Take molodan and observe under a microscope: the irregular branched mass is colorless and dissolves in chloral hydrate solution; the hyphae are colorless or light brown, with a diameter of 4-6 μm; the parenchyma cells are round and elliptical Shaped pits, integrated pit groups; non-glandular hairs T-shaped, head cells are fibrous; round cells on the fiber surface contain small round silicon blocks, arranged in rows; colorless irregular blocks are easy to see; wood Thrombus cells are dark yellow-brown, rectangular or long polygonal, and some cells are filled with yellow-brown contents and oil particles; stone cells are yellow-brown or colorless, sub-rectangular, sub-circular or irregular in shape, with a diameter of about 94 μm; pollen grains yellowish brown, sub-circular in shape, about 30 μm in diameter, with net-like carvings on the outer wall;
[0109] B. Take 9g of Molo...
Embodiment 2
[0118] Embodiment 2: the quality control method of Morodan
[0119] Identification method:
[0120] B. Take 10g of Molodan, cut it into pieces, add 11g of diatomaceous earth, grind it evenly, add 45ml of water, add 5ml of hydrochloric acid, extract under reflux for 30 minutes, filter, add 45ml of ethyl acetate to the filtrate each time, extract 4 times, and combine the solvents Ethyl acetate was evaporated to dryness, and the residue was dissolved in 1ml of chloroform as a test solution; another 1g of Ophiopogon japonicus reference drug was taken, and the reference drug solution was prepared in the same way; according to the thin-layer chromatography test, the above two solutions were absorbed Each 6 μl was spotted on the same silica gel G thin-layer plate, developed with 9:1 chloroform-acetone as the developer, taken out, dried in the air, sprayed with 9% sulfuric acid ethanol solution, and heated at 105°C until the spots became apparent. The color is clear; in the chromatog...
Embodiment 3
[0128] Embodiment 3: the identification method of Morodan:
[0129] B. Take 17g of Molodan, cut it into pieces, add 7g of diatomaceous earth, grind well, add 55ml of water, add 6ml of hydrochloric acid, extract by cold soaking for 30 minutes, filter, add 35ml of ethyl acetate to the filtrate each time, extract twice, and combine the solvents Ethyl acetate was evaporated to dryness, and the residue was dissolved by adding 1ml of chloroform, which was used as the test solution; another 1g of Ophiopogon japonicus reference drug was taken, and the reference drug solution was prepared in the same way; 9 μl, respectively spot on the same silica gel G thin-layer plate, use 5:4 chloroform-acetone as developer, develop, take out, dry in the air, spray with 11% sulfuric acid ethanol solution, heat at 105°C until the spots develop color Clear; in the chromatogram of the test product, there are spots of the same color at the position corresponding to the chromatogram of the control medici...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com