Preparation technology of 1-O-acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose
A technology of benzoyl and ribofuranose, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of high cost, high organic waste discharge, low product yield, etc., and achieve low cost , low content of nitrogen-containing organic matter, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
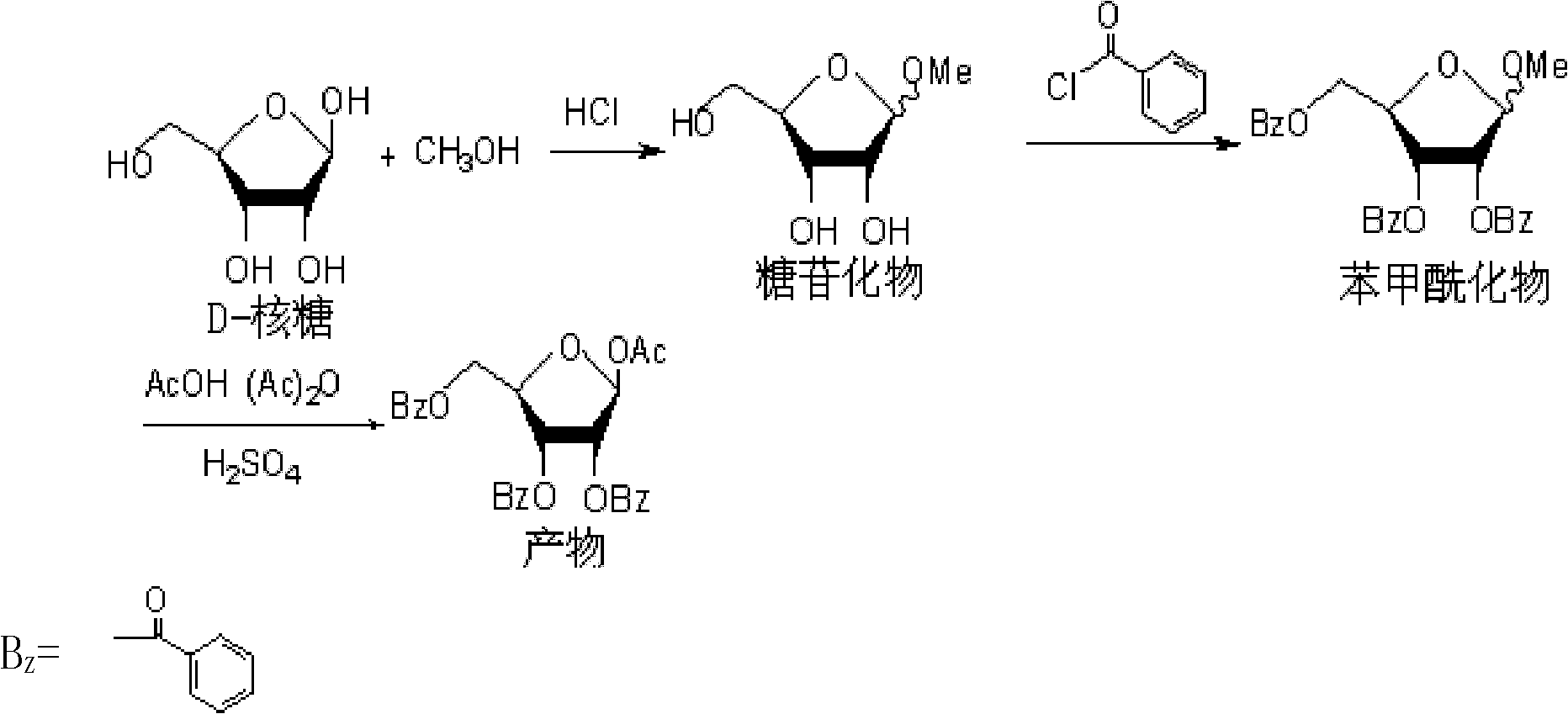
[0018] A preparation process for 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose, comprising the following steps:
[0019] (1) Glycosidation reaction Add 5ml of thionyl chloride dropwise to 100ml of methanol, cool in an ice-water bath, stir for 10-15 minutes, add 10g of D-ribose, and carry out glycosidation reaction at 0-5°C for 8 hours. Sodium neutralized the hydrogen chloride in the system, continued to stir for 30 minutes, filtered, concentrated the filtrate under reduced pressure to remove methanol, and obtained 11 g of the concentrate glycoside;
[0020] (2) Benzoylation reaction Mix 11 g of glycosidate with 150 ml of ethyl acetate and 5 ml of pyridine, heat to dissolve, add 30 g of potassium carbonate, and add 30 ml of benzoyl chloride dropwise at 60-70°C to carry out benzoylation reaction. After the dropwise addition, keep warm for 6 hours, filter out the insoluble matter in the system, wash the filtrate and combine, wash with water and 1.5mol / L sulfuric acid solution i...
Embodiment 2
[0024] A preparation process for 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose, comprising the following steps:
[0025] (1) Glycosidation reaction Add 5ml of thionyl chloride dropwise to 100ml of methanol, cool in an ice-water bath, stir for 10-15 minutes, add 10g of D-ribose, and carry out glycosidation reaction at 0-5°C for 8 hours. Sodium neutralized the hydrogen chloride in the system, continued to stir for 30 minutes, filtered, concentrated the filtrate under reduced pressure to remove methanol, and obtained 11 g of the concentrate glycoside;
[0026] (2) Benzoylation reaction Mix 11 g of glycosidate with 150 ml of ethyl acetate and 5 ml of pyridine, heat to dissolve, add 30 g of potassium carbonate, add 30 ml of benzoyl chloride dropwise at 40-50°C to carry out benzoylation reaction, Keep warm for 8 hours after the dropwise addition, filter out the insoluble matter in the system, wash the filtrate and combine, wash with water and 1.5mol / L sulfuric acid solution in tur...
Embodiment 3
[0030] A preparation process for 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose, comprising the following steps:
[0031] (1) Glycosidation reaction Add 5ml of thionyl chloride dropwise to 100ml of methanol, cool in an ice-water bath, stir for 10-15 minutes, add 10g of D-ribose, and carry out glycosidation reaction at 0-5°C for 8 hours. Sodium neutralized the hydrogen chloride in the system, continued to stir for 30 minutes, filtered, concentrated the filtrate under reduced pressure to remove methanol, and obtained 11 g of the concentrate glycoside;
[0032] (2) Benzoylation reaction Mix 11g of glycosidate with 150ml of dichloroethane and 5ml of pyridine, heat to dissolve, add 30g of potassium carbonate, drop 30ml of benzoyl chloride at 40-50°C to carry out benzoylation reaction , keep warm for 6 hours after the dropwise addition, filter out the insoluble matter in the system, wash the filtrate and combine, wash with water and 1.5mol / L sulfuric acid solution successively, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com