Cell membrane imaging fluorescence probe and application thereof
A technology for fluorescent probes and cell imaging, which is applied in fluorescence/phosphorescence, luminescent materials, color/spectral characteristic measurement, etc., can solve problems such as poor stability and fluorescence quenching, and achieve good acid resistance, light stability, and stable fluorescence performance , The effect of stable fluorescence imaging performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment proposes a cell membrane fluorescent probe containing pyranoside perylene imide derivatives, N -n-octyl- N’ -((4-Aminophenyl)- β -D-glucopyranoside)-3,4:9,10-peryleneimide, referred to as: PDI-OBAG, has the following molecular structure:
[0044]
[0045] The preparation method of fluorescent probe is as follows:
[0046] Add 0.5 g (0.99 mmol) N-octyl-3,4:9,10-perylenetetracarboxylic acid-3,4-anhydride-9,10-imine into a 100 mL round-bottomed flask, and add 0.35 g (4-aminophenyl)-β-D-glucopyranoside, 1.09 g zinc acetate, 15.0 g imidazole, under nitrogen protection, react at 160 °C for 2.5 h. After the reaction was stopped, cool to room temperature, transfer the reactant to a mixed solution of 480 mL of ethanol and 80 mL of water, stir, let it stand overnight, and filter with suction to obtain a red solid, which was dried in vacuo, and then washed with CH 2 Cl2 and a small amount of DMF washing, get N -n-octyl- N’ -((4-Aminophenyl)- β -D-glucopyr...
Embodiment 2
[0048] Application of cell membrane fluorescence imaging based on peryleneimide derivatives described in Example 1:
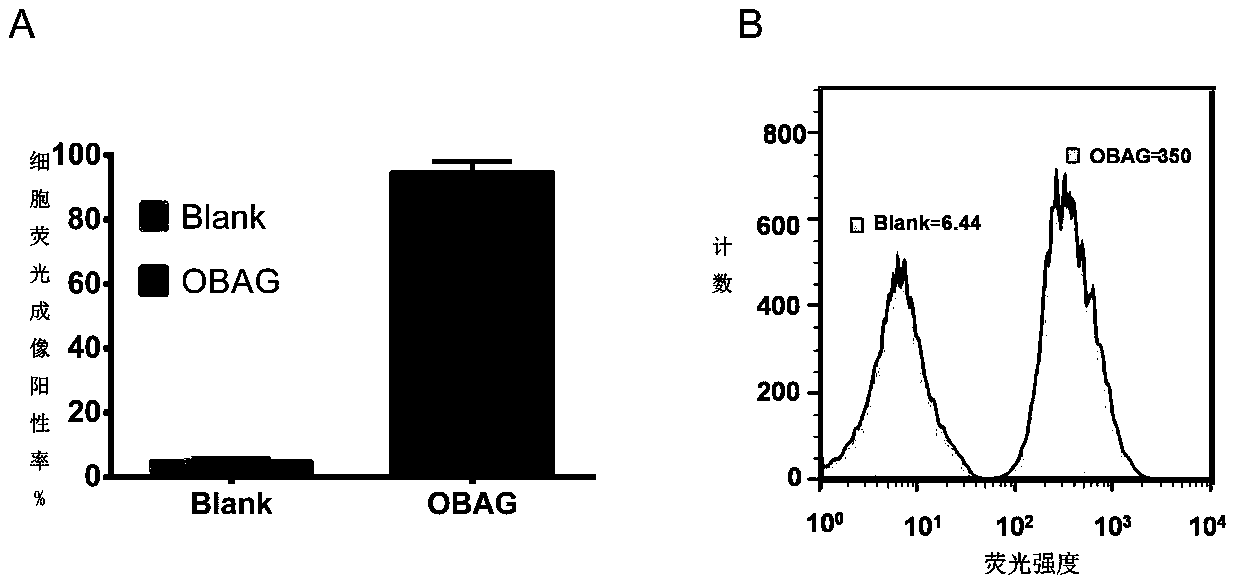
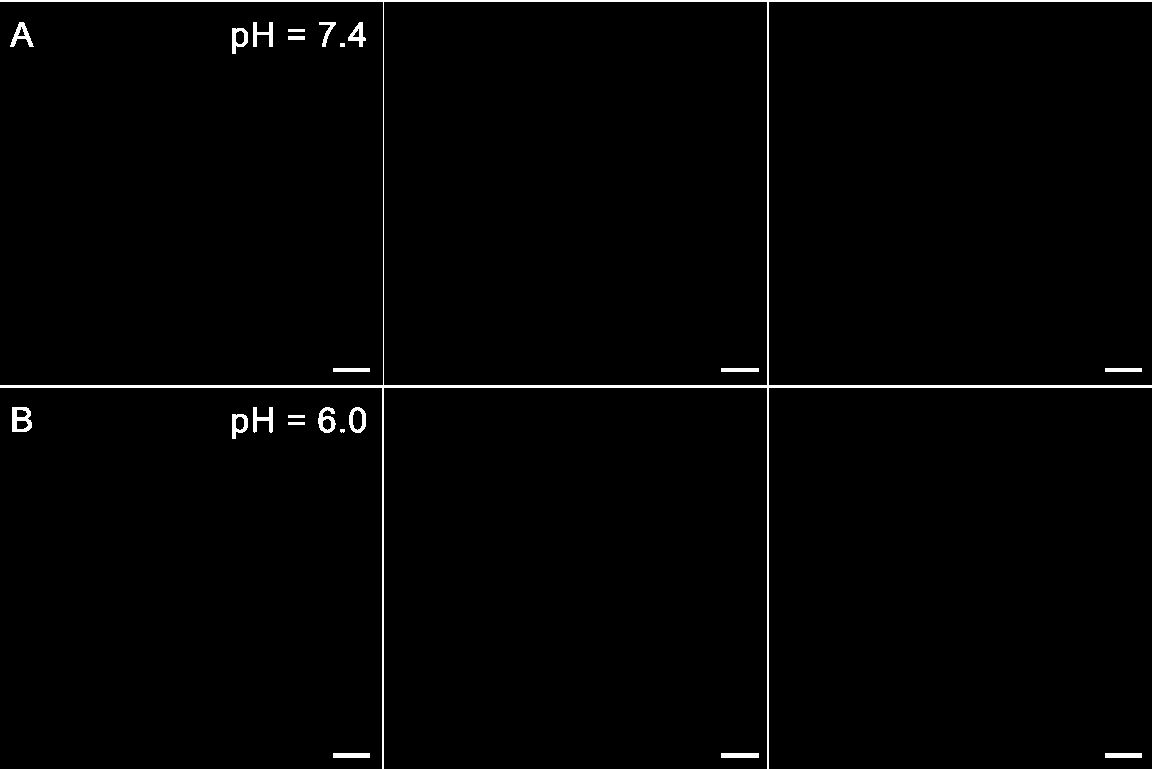
[0049] 1) Dissolving PDI-OBAG in PBS to obtain a working solution containing a fluorescent probe, the concentration of the fluorescent probe in the working solution is 10 μM;
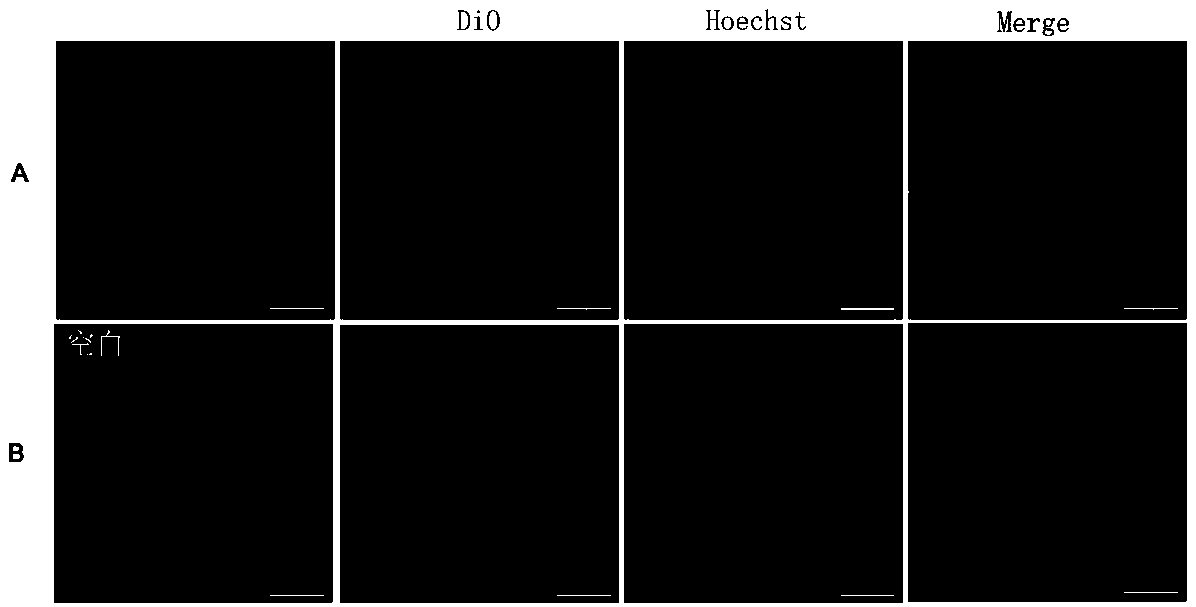
[0050] 2) Use the working solution described in step 1) to incubate with SH-SY5Y cells for 25-35 min, and form a cell image after excitation; the wavelength of the excitation light is 605 nm.
[0051] Specific steps are as follows:
[0052] 1. The culture process of the SH-SY5Y cells:
[0053] 1. Recovery of SH-SY5Y cells
[0054] (1) Quickly place the cryopreservation tube of SH-SY5Y cells in warm water at 37 ℃, shake gently to melt it as soon as possible, and take out the cryopreservation tube after 30 seconds in the water bath;
[0055] (2) After sterilizing the outer surface of the cryovial with 75% alcohol, use a straw to suck out the cell suspension in the cryovial and pour it i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com