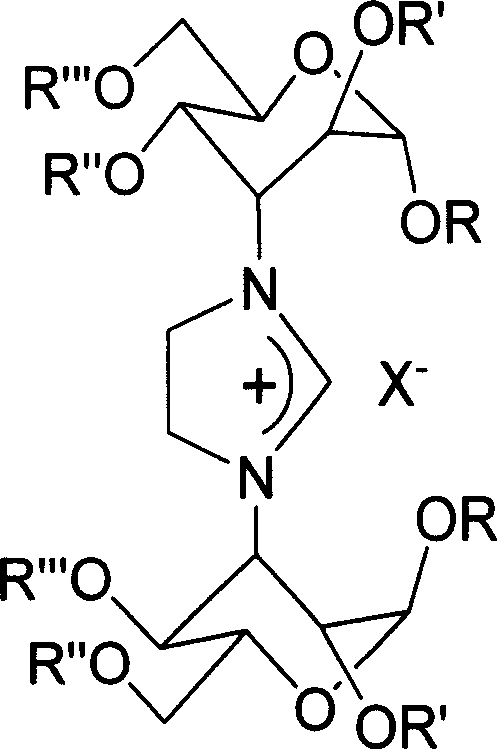
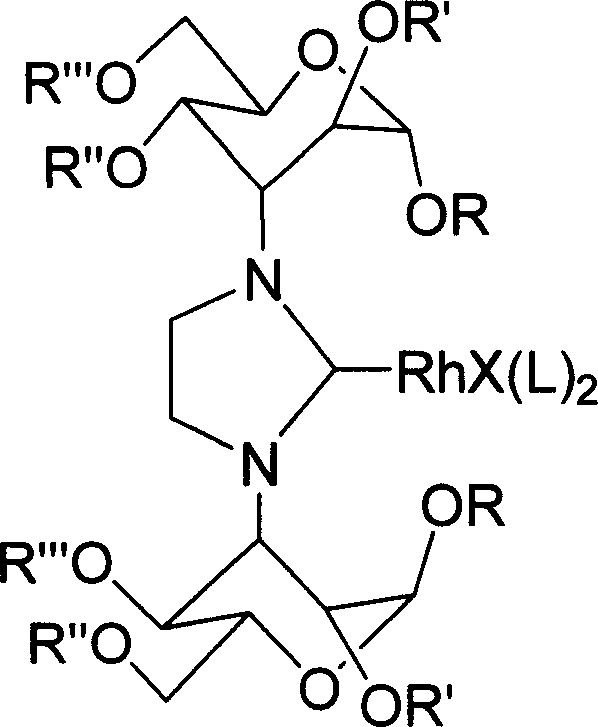
Imidazoline salt, rhodium complex of carbohydrate derivative and its preparation method
A technology of carbohydrates and rhodium complexes, applied in organic compound/hydride/coordination complex catalysts, sugar derivatives, chemical instruments and methods, etc., can solve problems such as chiral imidazoline salts that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] At 110°C, N,N'-bis(methyl 3-deoxy-4,6-O-benylidene-α-D-altropyranoside-3-yl)ethylenediamine (2.0g, 3.4mmol ), ammonium chloride (0.3g, 5.6mmol) was reacted in 40mL of triethyl orthoformate for 3 hours, cooled, filtered, and washed with petroleum ether to obtain 2.0g product (92%); m.p.153-4°C; [a ] 20 D =+127.9 (c 1.0, ethanol). 1 H NMR (400MHz, CDCl 3 ): δ 8.83(s, 1H), 7.40-7.15(m, 10H), 5.88(br, 2H), 5.50(s, 2H), 4.57(s, 2H), 4.30-4.05(m, 8H), 3.90 (br, 2H), 3.75-3.60(m, 4H), 3.10(s, 6H), 3.06(s, 2H). 13 C NMR (100MHz, CDCl 3 ): δ 157.29, 136.96, 129.44, 128.45(2C), 126.12(2C), 102.55, 100.70, 74.51, 69.30, 66.86, 58.66, 58.22, 55.43, 50.09. IR(KBr): 3440, 3257, 29230, 16 1459, 1409, 1262, 1138, 1104, 995, 964, 772, 707. MS (ESI) m / z (%): 599 [M + -Cl] (100).
Embodiment 2
[0025] At 45°C, the product (1.35g, 2.15mmol) obtained in Example 1, [RhCl(COD)] 2 (0.40g, 0.82mmol) and NaO t Bu (0.31g, 3.2mmol) was reacted in THF (20mL) for 48 hours, filtered and purified by column chromatography to obtain 1.06g product (77%). m.p.157-9℃. 1 H NMR (400MHz, CDCl 3 ): δ7.60-7.20(m, 11H), 6.60-6.62(m, 1H), 5.80(br, 1H), 5.71(s, 1H), 5.64(s, 1H), 5.05-4.90(m, 2H ), 4.69(d, J=3.2Hz, 1H), 4.66(s, 1H), 4.42(br, 1H), 4.35-4.25(m, 3H), 4.11(br, 2H), 4.05-3.98(m, 1H), 3.92-3.81(m, 2H), 3.80-3.65(m, 5H), 3.64-3.55(m, 2H), 3.46(s, 3H), 3.39(br, 1H), 3.27(s, 3H) , 2.55-2.42(m, 2H), 2.35-2.22(m, 2H), 2.10-1.98(m, 2H), 1.82-1.67(m, 4H). 13 C NMR(100MHz,CDCl3):δ 137.65,137.42,129.00,128.56,128.40,128.04,126.30,126.01,102.31,101.40,101.21,74.70,73.80,71.67,70.22,69.94,69.04,68.90,62.41,61.72,59.38 , 58.52, 55.48, 55.09, 49.23, 47.41, 34.18, 31.68, 29.96, 27.96. 689. MS (ESI) m / z (%): positive 809 [M + -Cl](100); negative 879[M + -Cl](100).HRMS m / z Calc.for C 39 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com