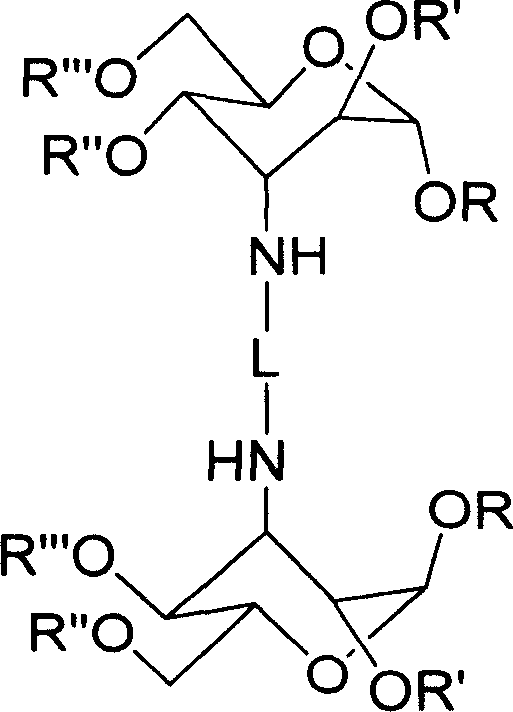
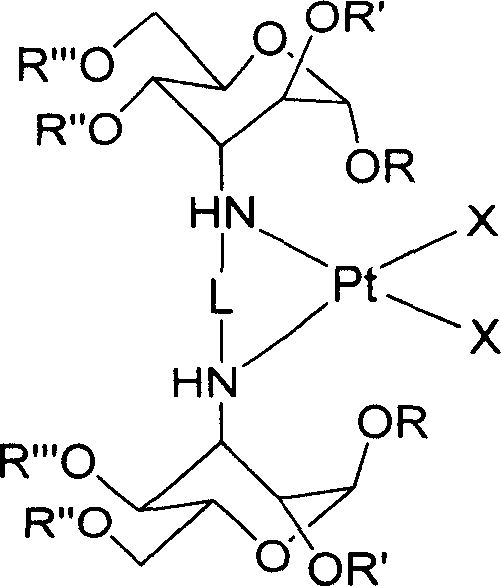
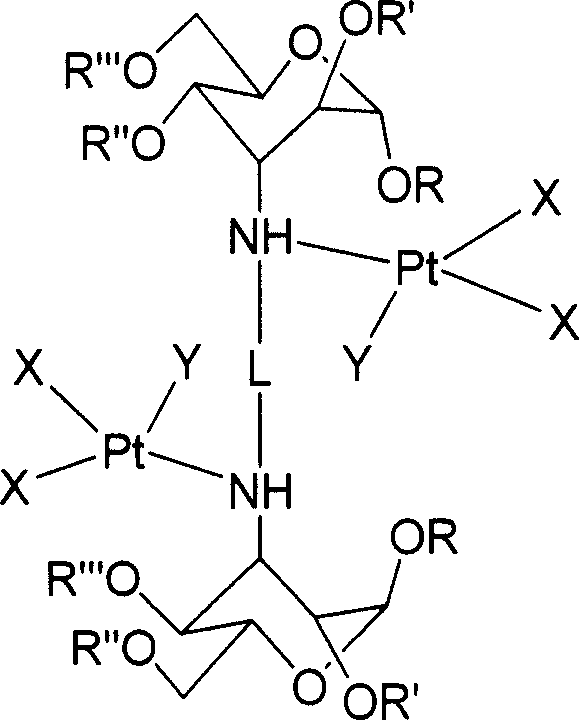
Carbohydrate derivative, platinum complex with antitumour activity and its preparation method
A technology of carbohydrates and platinum complexes, applied in the direction of sugar derivatives, sugar derivatives, chemical instruments and methods, etc., can solve the problem of reducing drug activity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Ethylenediamine (0.40g, 6.7mmol), methyl 2,3-anhydride-4,6-O-benylidene-α-D-mannopyranoside (4.09g, 15.5mmol) and K 2 CO 3 (0.2g) was reacted in 50mL of DMSO at 105-135°C for 10 hours, the solvent was removed under reduced pressure, and the residue was purified by column chromatography to obtain N,N-bis(methyl 3-deoxy-4,6-O- Benylidene-[alpha]-D-altropyranoside-3-yl)ethylenediamine 3.39 g (86%). m.p.91-3°C; [a] 21 D =+112.2(c1.0, ethanol). 1 H NMR (400MHz, CDCl 3 ): δ7.50-7.30(m, 10H), 5.47(s, 2H), 4.52(s, 2H), 4.30-4.25(m, 2H), 4.10-4.00(m, 2H), 3.95-3.90(m , 2H), 3.91(br, 2H), 3.80-3.70(m, 2H), 3.34(s, 6H), 3.22(br, 2H), 3.10-3.05(m, 2H), 2.90-2.85(m, 2H ), 2.65-2.55 (m, 2H); 13 CNMR (100MHz, CDCl 3 ): δ137.64 129.06 128.38(2C), 126.21(2C), 102.05(2C), 77.86, 70.80, 69.50, 58.69, 57.10, 55.47, 47.08, 46.11. IR(KBr): 3440, 2907, 1637, 1457, 1380, 1105, 1067, 1044, 974, 755, 670. MS (ESI) m / z (%): 589 [M + +1] (100).
Embodiment 2
[0036] Butanediamine (0.79g, 9.0mmol), methyl 2,3-anhydride-4,6-O-benylidene-α-D-mannopyranoside (5.28g, 20.0mmol) and K 2 CO 3 (0.2g) was reacted in 50mL of DMSO at 120°C for 10 hours, the solvent was removed under reduced pressure, and the residue was purified by column chromatography to obtain N,N-bis(methyl 3-deoxy-4,6-O-Bian Alkyne-[alpha]-D-altropyranoside-3-yl)butanediamine 5.10 g (92%). m.p.124-6°C; [a] 21 D =+131.6(c1.0, ethanol). 1 H NMR (400MHz, CDCl 3 ): δ7.55-7.30(m, 10H), 5.44(s, 2H), 4.56(s, 2H), 4.30-4.25(m, 2H), 4.15-4.00(m, 2H), 3.95-3.85(m , 4H), 3.85-3.70(m, 2H), 3.35(s, 6H), 3.33(br, 2H), 3.15-3.05(m, 2H), 2.90-2.85(m, 2H), 2.65-2.55(m , 6H).IR (KBr): 3442, 2911, 1635, 1457, 1376, 1101, 1063, 1045, 976, 753, 674. MS (ESI) m / z (%): 617 [M + +1] (100).
Embodiment 3
[0038] Hexamethylenediamine (0.71g, 6.1mmol), methyl 2,3-anhydride-4,6-O-benylidene-α-D-mannopyranoside (3.96 g, 15.0mmol) and K 2 CO 3 (0.3g) was reacted in 50mL of DMSO at 120°C for 10 hours, the solvent was removed under reduced pressure, and the residue was purified by column chromatography to obtain N,N-bis(methyl 3-deoxy-4,6-O-Bian Alkyne-[alpha]-D-altropyranoside-3-yl)hexamethylenediamine 3.34 g (85%). m.p.161-4°C; [a] 21 D =+127.6(c1.0, ethanol). 1 H NMR (400MHz, CDCl 3): δ7.55-7.30(m, 10H), 5.43(s, 2H), 4.52(s, 2H), 4.30-4.25(m, 2H), 4.10-4.00(m, 2H), 3.95(br, 2H ), 3.85-3.70(m, 2H), 3.33(br, 2H), 3.15-2.55(m, 14H).IR(KBr): 3440, 2908, 1633, 1458, 1375, 1106, 1063, 1044, 977, 753, 675. MS (ESI) m / z (%): 645 [M + +1] (100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com