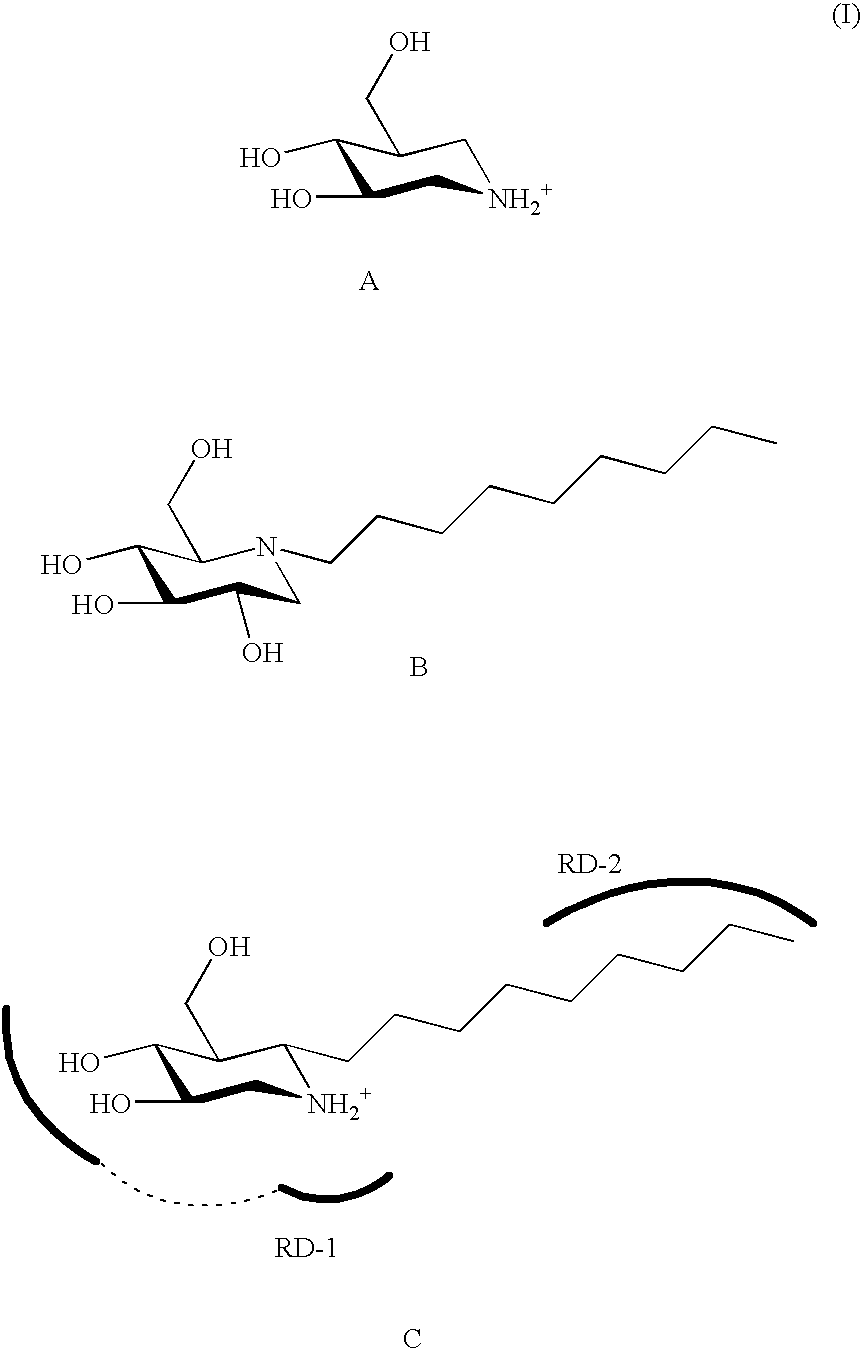
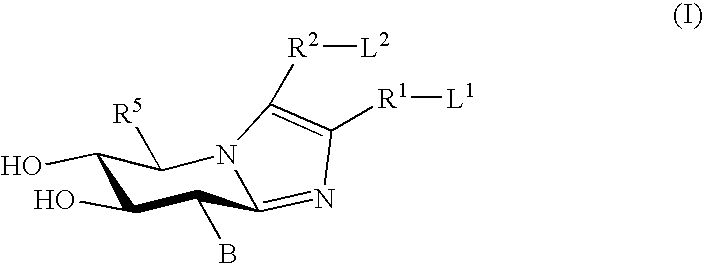
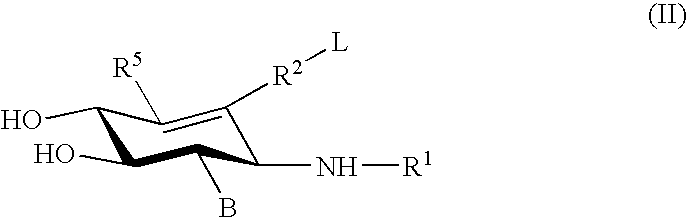
Glucoimidazole and polyhydroxycyclohexenyl amine derivatives to treat gaucher disease
a technology of glucoimidazole and phca, which is applied in the field of glucoimidazole (giz) and phca derivatives, can solve the problems of lack of specificity, difficult or inappropriate clinical use, complex protein folding, etc., and achieve the effect of enhancing the activity of gcas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of GIZ Derivatives
a. (5R,6R,7S,8S)-6,7,8-Tris(benzyloxy)-5-[(benzyloxy)methyl]-2-(1-octynyl)-5,6,7,8-tetrahydroimidazo[1,2-a]pyridine
[0177] (5R,6R,7S,8S)-6,7,8-Tris(benzyloxy)-5-[(benzyloxy)methyl]-2-iodo-5,6,7,8-tetrahydroimidazo[1,2-a]pyridine as described by Panday in Helv. Chim. Acta., 83, 58-79 (2000), (300 mg, 0.437 mmol) is combined with [Pd(PPh3)4] (0.252 mg, 0.22 mmol), Et3N (0.27 ml), CuI (8 mg, 0.04 mmol), and 1-octynye (0.19 ml, 1.31 mmol) in DMF (7.5 ml) and is stirred at 80° C. for 7 hours. The reaction mixture is diluted with ether (100 ml) and washed with water and then aq. sat. NaCl. The organic layer is dried over Na2SO4 and evaporated using a rotovap. Purification by flash chromatography using CH2Cl2 as the eluent gives the title compound as brown syrup. 1H NMR (300 MHz, CDCl3): δ7.43-7.14 (m, 21H), 5.15 (d, 1H, J=12 Hz), 4.86-4.75 (m, 4H), 4.62 (d, 1H, J=11.4 Hz), 4.50-4.44 (m, 3H), 4.20-4.16 (m, 1H), 4.10-4.07 (m, 1H), 3.84-3.78 (m, 2H), 3.73-3.70 (...
example 2
Inhibitory Activities of Glucoimidazole Derivatives Against GCase
[0181] Methods. The enzyme activity was assayed with 4-methylumbelliferyl β-glucoside (final concentration 3 mM) as substrate in Reaction Buffer consisting 0.25% sodium taurocholate, 0.1% Triton X-100 in McIlvaine buffer (0.1M citrate and 0.2M phosphate buffer) at pH 5.2. The compounds were added to each reaction mixture individually at the final concentration indicated. After incubation with the diluted human GCase at 37° C. for 30 min, the reaction was stopped by addition of 0.2 M glycine buffer, pH 10.8, the released 4-methylumbelliferone was determined by a fluoremeter at excitation wavelength of 355 nm and emission wavelength at 460 nm, respectively. The relative enzyme activity was calculated as a percentage to those of reactions without inhibitors. IC50 was calculated using Prism sigmoid plot.
[0182] Results. GIZ and 2-PE-GIZ were reported to be potent inhibitors for β-glucosidases (Panday et al., Helvetica Chi...
example 3
Chaperone Activity of GIZ for GCase in Gaucher Cells
[0183] Methods. Fibroblasts established from Gaucher patients with N370S / N370S mutation were cultured in DMEM medium supplemented with 10% fetal bovine serum and antibiotics at 37° C. under 5% CO2. The GIZ was added into the culture medium at the final concentrations for 4 days prior to the assay. After washing the cells with phosphate-buffered saline, the cells were harvested and homogenized in the presence of 0.25% (w / v) sodium taurocholate and 0.1% (v / v) Triton X-100 in McIlvaine buffer (pH 5.2, Reaction Buffer), and 10 μl of the lysate was used for the determination of residual enzyme activity. The activity of the GCase was determined with 3 mM 4-MU-β-Glc in the Reaction Buffer at 37° C. for 1 hr as conduritol B epoxide (CBE)-sensitive activity in parallel assays without or with pre-incubation with CBE at 1.25 mM for 30 min at room temperature. Protein concentrations in the cell lysates were determined using micro BCA protein ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| mass densities | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com