Construction method of <1>H-NMR fingerprint of radix astragali injection
A technology of astragalus injection and fingerprints, which is applied in the field of quality control of traditional Chinese medicine injections, and can solve problems such as difficult detection of chromatographic fingerprints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Astragalus Injection 1 Establishment of H-NMR Fingerprint
[0024] 1) Sample preparation:
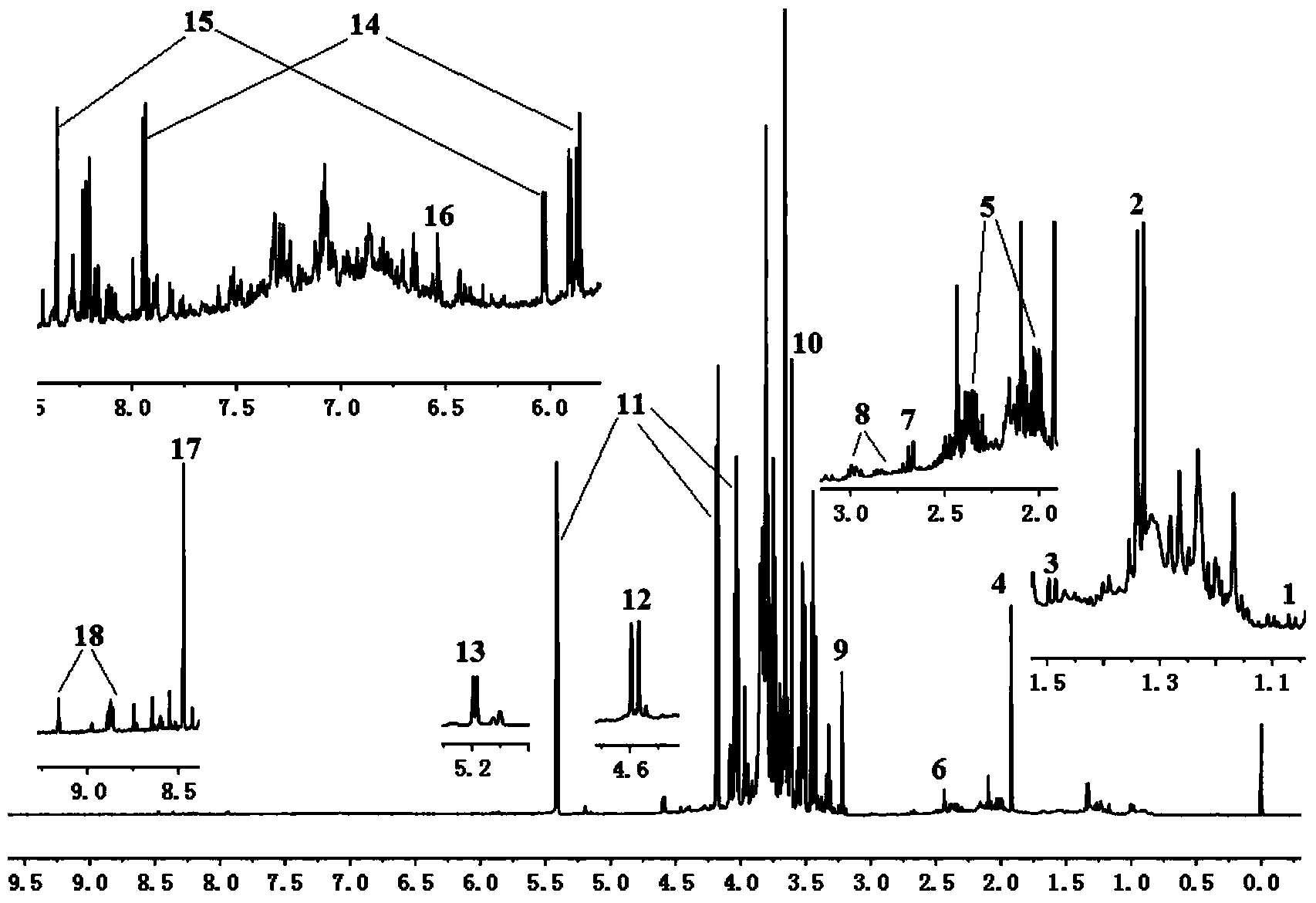
[0025] ① Take 10ml of Astragalus injection and directly evaporate to dryness, take 50mg of dry powder, dissolve with deuterated methanol: heavy water = 1:1, transfer to a 1.5ml centrifuge tube, centrifuge at 13000rpm for 10 minutes, pipette 600μL of supernatant into a 5mm NMR tube , for Astragalus Injection 1 H-NMR fingerprint spectrum A analysis;
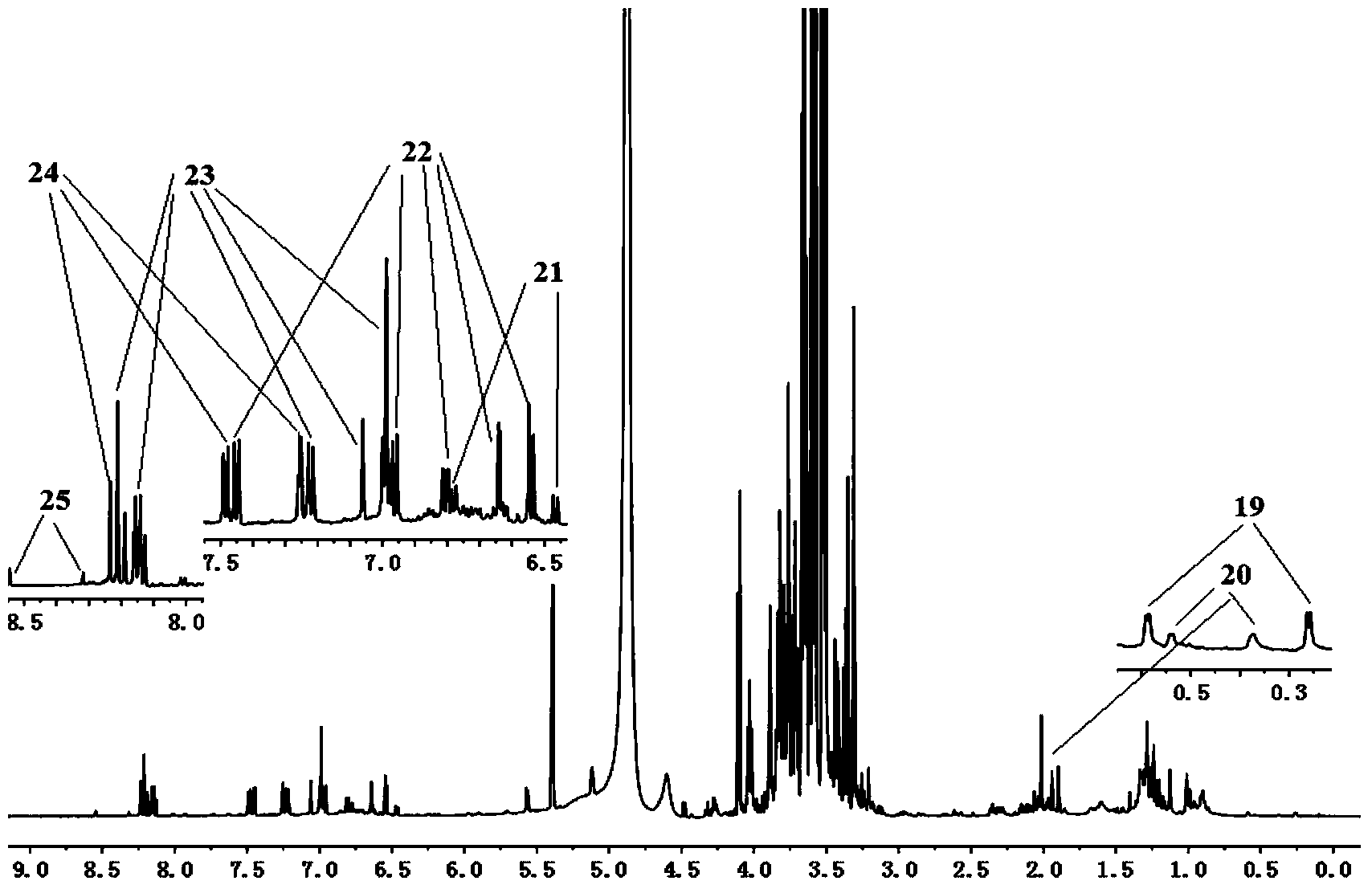
[0026] ② Take 30ml of Astragalus injection, add an equal volume of ethyl acetate to extract twice, combine the extracts, evaporate to dryness, add 800μL of deuterated methanol to dissolve, transfer to a 1.5ml centrifuge tube, centrifuge at 13000rpm for 10 minutes, and pipette 600μL of supernatant The solution is used in a 5mm NMR tube for Astragalus Injection 1 H-NMR fingerprint spectrum B analysis;
[0027] 2) Spectrum collection: The sample is measured on a 600MHz NMR instrument at 25°C, and the measurement frequen...
Embodiment 2
[0031] Embodiment 2 This method is used for the quality evaluation of Radix Astragali Injection
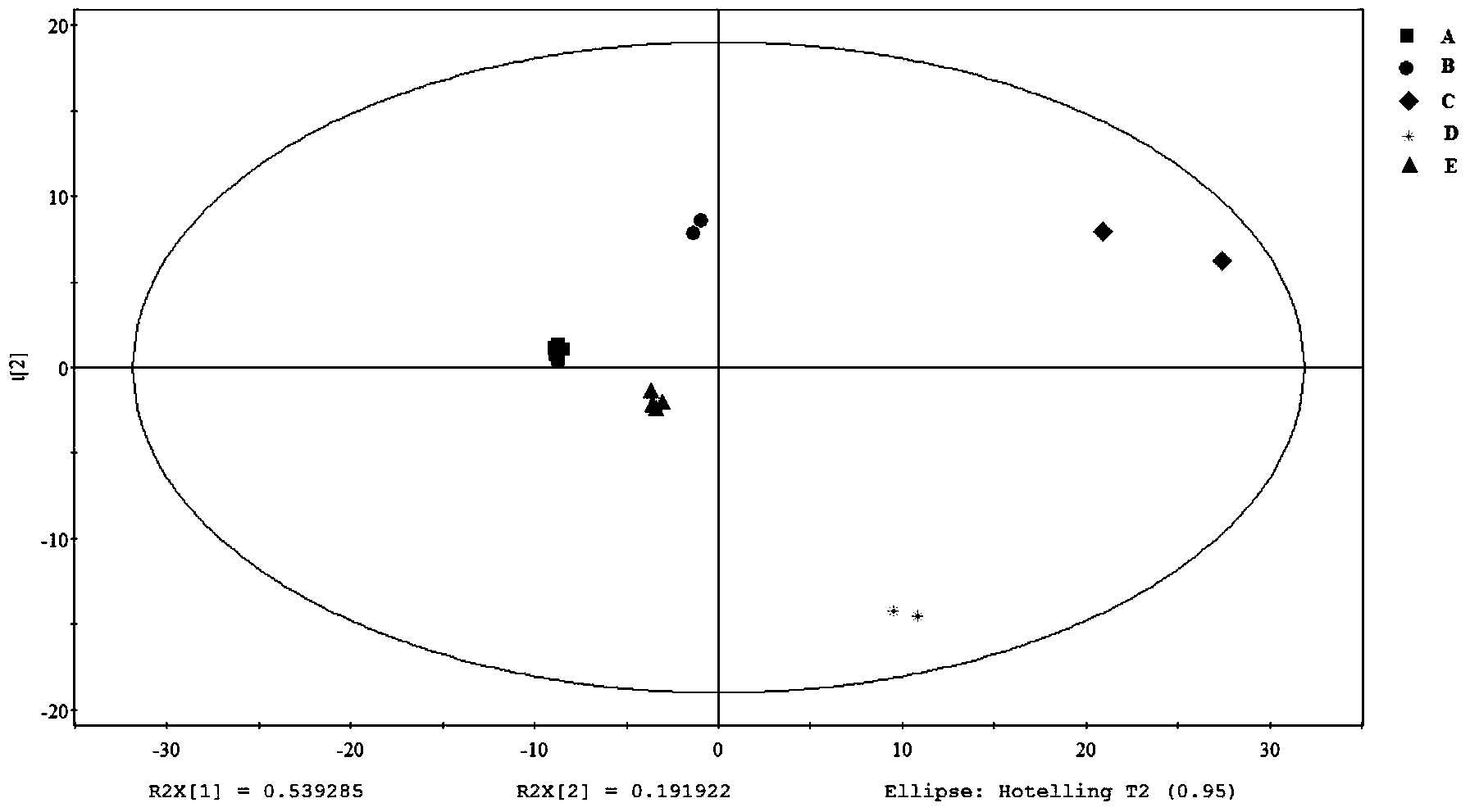
[0032] A total of 8 batches of Astragalus injection from 5 different manufacturers (denoted as: A, B, C, D, E) were collected (A manufacturer: 3 batches; B manufacturer: 1 batch; C manufacturer: 1 batch D producer: 1 batch; E producer: 2 batches), carry out according to the method determined in the content of the present invention 1 H-NMR analysis, obtained different manufacturers of Astragalus injection 1 H-NMR fingerprints A and B; segmented integration of fingerprint A (chemical shift range 0.34-10.02) and fingerprint spectrum B (chemical shift range 0.76-9.92) with δ0.04ppm, where δ4.80~5.06 (residual water peak) and δ3.30~3.34 (residual methanol peak) are not integrated, and the integrated data is imported into SIMCA-P 11.0 software for PCA (principal component analysis) and PLS-DA (partial least squares discriminant multiplication) analyze.
[0033] Astragalus Injection ...
Embodiment 3
[0034] Example 3 Relative Content Comparison of Primary Metabolites of Astragalus Injection from Different Manufacturers
[0035] Astragalus Injection 1 The H-NMR fingerprint spectrum A was analyzed, with TSP as the internal standard, and the relative content of 13 primary metabolites was calculated by the integral area. From Figure 5 It can be seen that there are large differences in the relative content and proportion of primary metabolites among different manufacturers. There are fewer types of primary metabolites in manufacturers B and C, and the relative content and proportion of 13 primary metabolites in different batches of Astragalus injection from the same manufacturer. The content and proportion are close. From Figure 6 It can be seen that the same primary metabolites are different among different manufacturers and different batches of the same manufacturer. It can be seen from this example that NMR fingerprints can be used to compare the homogeneity of primary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com