Crucian herpes virus disease compound vaccine preparation, preparation method and application
A crucian carp herpes virus and preparation technology, applied in antiviral agents, biochemical equipment and methods, viral peptides, etc., can solve the problems of high-efficiency expression of heterogeneous biological cells, difficulty of exogenous genes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Acquisition of JDORF25, JDORF25C, JDORF25D gene encoded proteins:
[0069] 1. Truncated region screening
[0070] The antigenic region, hydrophilic region and surface display probability of ORF25, ORF25C, ORF25D genes of carp herpesvirus type II were analyzed, and the hydrophilic sequences with concentrated antigenic determinants and strong antigenicity were selected as the proposed expression genes.
[0071] 2. Codon optimization
[0072] According to the yeast codon preference, codon optimization was performed on the proposed expression gene sequence, and the GC content was kept moderate, and the optimized sequence was synthesized. Compared with the original sequence of the optimized ORF25, ORF25C, ORF25D gene (JDORF25, JDORF25C, JDORF25D) coding sequence, 20%-30% of the bases in the sequence are optimized and the low-frequency codons of Pichia pastoris are all replaced by high-frequency or sub-high frequency codons.
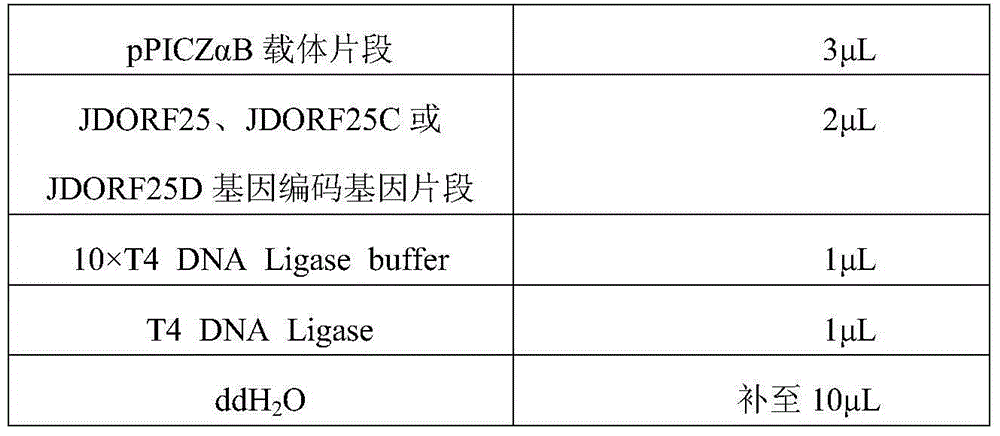
[0073] 3. Amplification of JDORF25, JDORF25C, ...
Embodiment 2
[0121] A preparation method of crucian carp herpes virus disease vaccine, the steps are as follows:
[0122] Pick Pichia pastoris Km71 / CyHV-2-25, Pichia pastoris Km71 / CyHV-2-25C, and Pichia pastoris Km71 / CyHV-2-25D strains, and inoculate them into 100mL BMGY culture medium, 30°C, 250r / min shaking culture to OD 600 When the value reaches about 6, centrifuge at room temperature at 1500g for 5min, resuspend the bacteria in 20mL of BMMY medium, transfer the obtained bacterial solution into a 100mL shake flask, seal the bottle, add 100% methanol to a final concentration of 0.5%, and store at 30°C, 250r / The culture was induced under the condition of 1 min, and methanol was added every 24 hours to maintain the concentration of methanol at 0.5%. After continuous induction for 3 days, the yeast in the induction solution was removed by centrifugation (removing the precipitate and leaving the supernatant), and the content of the target protein (recombinant protein JDORF25, JDORF25C, JD...
Embodiment 3
[0127] The application of a crucian carp herpes virus disease compound vaccine preparation in the preparation of carp herpes virus type II vaccine is as follows:
[0128] The vaccine prepared in Example 2 was used to immunize crucian carp (250 ± 20 grams) with basically no difference in body weight, good mental condition, and negative carp herpes virus type II, and a positive control and a normal saline control (100 microliters of each crucian carp intramuscularly injected, 200 microliters, 300 microliters, the specific concentration is shown in Table 1), after 15 days after boosting immunization with the same dose, each fish was intraperitoneally injected with 500 microliters of 1×10 6 . 5 TCID 50 / ml of carp herpesvirus type Ⅱ for challenge experiments to detect whether the antibodies produced are protective.
[0129] Table 1 Grouping of crucian carp herpes virus disease yeast immune protection experiment
[0130] test group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com