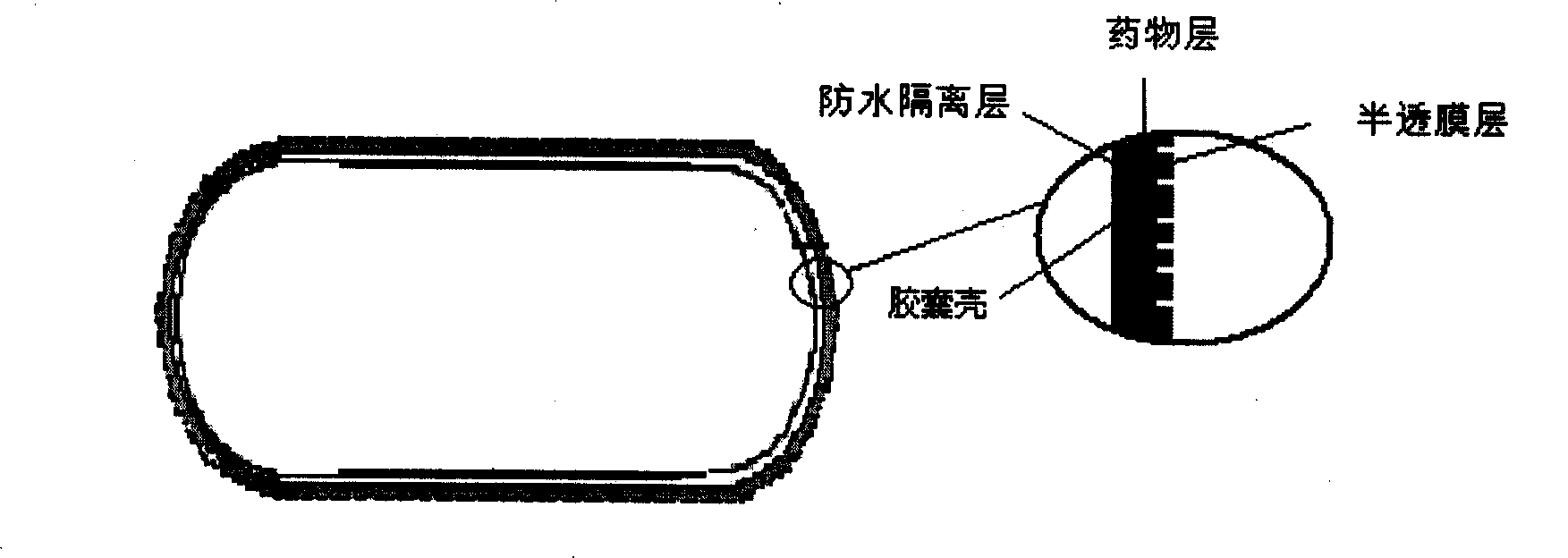
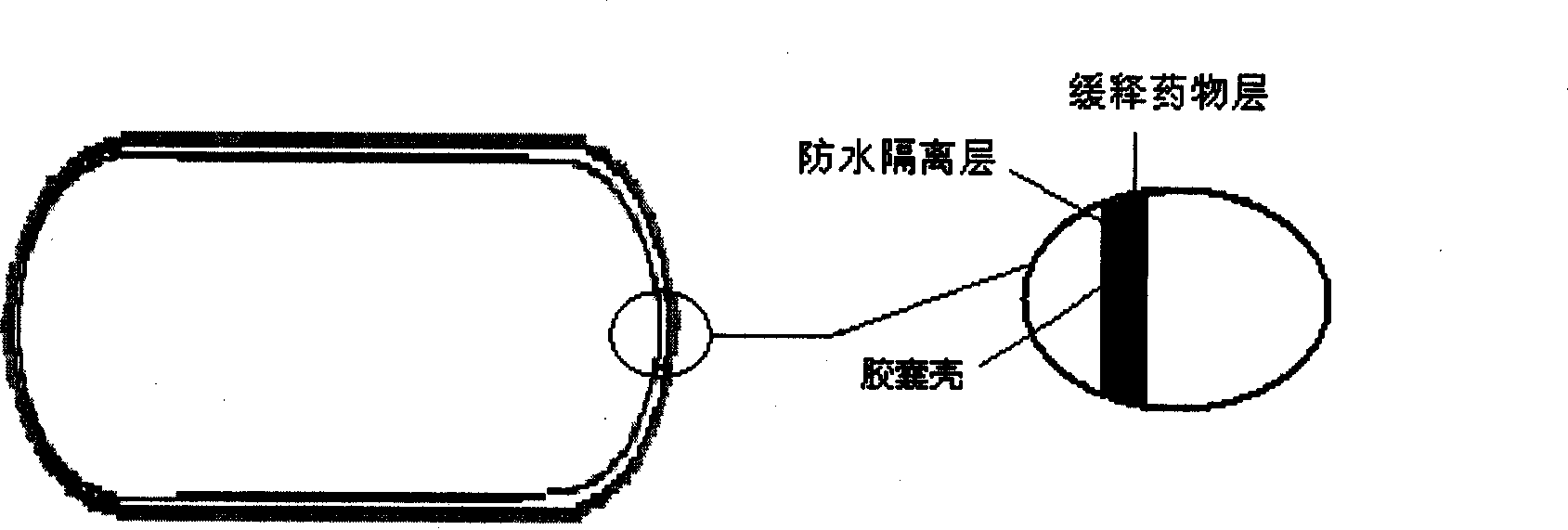
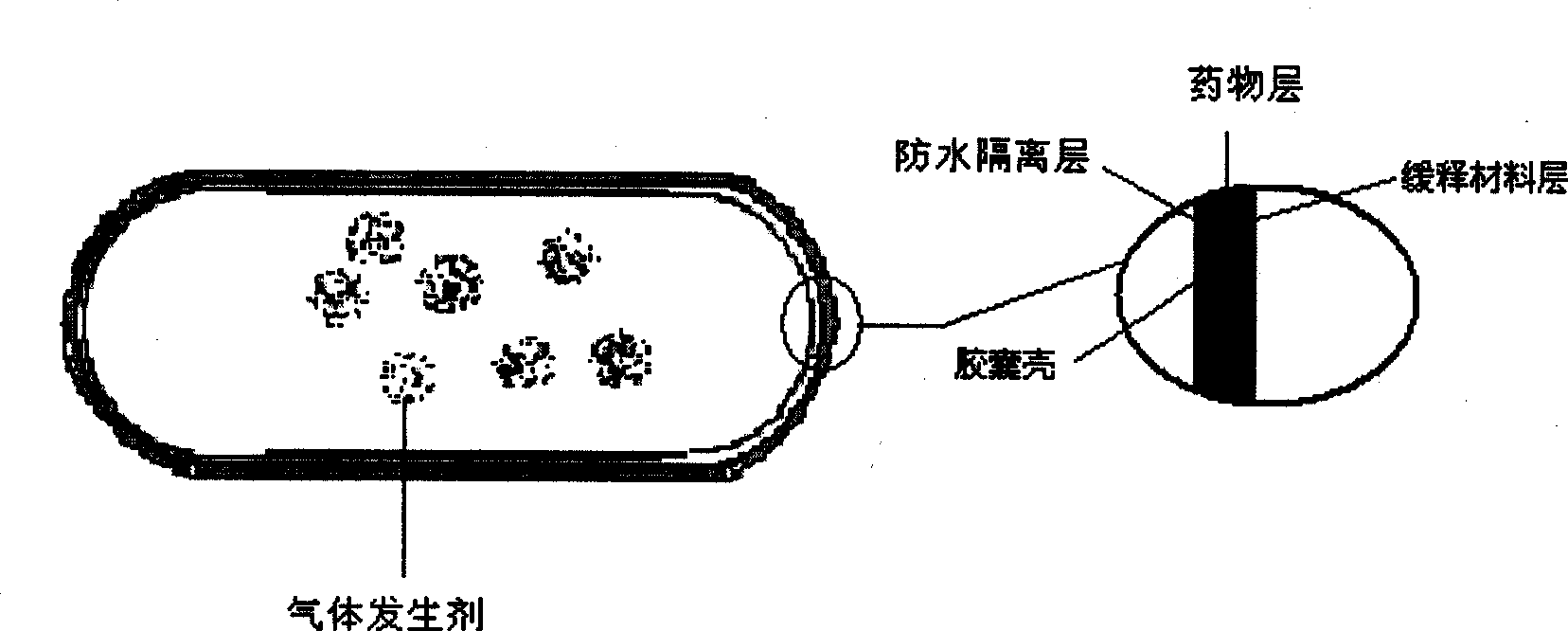
Stomach detention sustained and controlled release medicament releasing system and preparation method
A technology for controlled-release drugs and sustained-release drugs, applied in the field of medicine, can solve the problems of using many excipients, limiting flexibility, and high density of tablet cores.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Air bag composition: Sustained release layer composition:
[0102] Capsule No. 1 1000 Rosiglitazone Tartrate 8g
[0103] Ethyl cellulose 9.0g Stearic acid 32g
[0104] Stearic acid 15.0g Ethyl cellulose 40g
[0105] Triethyl citrate 4.5g Povidone k30 16g
[0106] Talc powder 3.0g Polyethylene glycol 24g
[0107] Add absolute ethanol to 300ml Talc powder 16g
[0108] 90% ethanol solution to 1000ml
[0109] Process: Put the No. 1 capsule shell into the coating pan, spray the prepared waterproof coating solution (dissolved / dispersed by stearic acid, ethyl cellulose, triethyl citrate, talc powder) at a tablet bed temperature of 45°C Prepared in absolute ethanol), after the weight gain of the coating was 15%, the capsule was locked, and the weight gain of the coating was 15%, and placed at 45°C for 12h to obtain a waterproof air bag; Rosiglitazone tartrate, stearic acid , ethyl cellulose, povidone, polyethyle...
Embodiment 2
[0111] Air bag composition: Drug layer:
[0112] Capsule No. 1 1000 pcs Rosiglitazone Maleate 1.5g
[0113] Ethylcellulose 9.0g Eudragit RL100 4.5g
[0114] Stearic acid 15.0g Ethyl cellulose 2.0g
[0115] Triethyl citrate 4.5g Polyethylene glycol 1.0g
[0116] Talc powder 3.0g 90% ethanol solution 100ml
[0117] Add absolute ethanol to 300ml
[0118] Isolation layer: Controlled release coating:
[0119] Lactose 30g Eudragit RS100 2.5g
[0120] povidone k30 3.0g Ethylcellulose 2.0g
[0121] Absolute Ethanol 200ml Polyethylene Glycol 1.0g
[0122] Triethyl citrate 0.8g
[0123] 90% ethanol 100ml
[0124] Process: Put the No. 1 capsule shell into the coating pan, spray the prepared waterproof coating solution (dissolved / dispersed by stearic acid, ethyl cellulose, triethyl citrate, talc powder) at a tablet bed temperature of 45°C prepared in absolute ethanol), after the weight gain of coati...
Embodiment 3
[0126] Airbag Composition Eudragit RL100 4.5g
[0127] Waterproof layer: ethyl cellulose 2.0g
[0128] Eudragit L100 9.0g Polyethylene glycol 1.0g
[0129] Stearic acid 15.0g 90% ethanol solution 100ml
[0130] Triethyl citrate 4.5g
[0131] Talc powder 3.0g Controlled Release Coating:
[0132] Absolute ethanol to 300ml Eudragit RS100 2.5g
[0133] Ethylcellulose 2.0g
[0134] Composition of drug-containing layer: Polyethylene glycol 1.0g
[0135] Drug layer: Triethyl citrate 0.8g
[0136] Rosiglitazone Maleate 1.5g 90% Ethanol 100ml
[0137] Process: Put the No. 1 capsule shell into the coating pan, spray the prepared waterproof coating solution (dissolved / dispersed in stearic acid, Eudragit L100, triethyl citrate, talc powder in the water ethanol), after the weight gain of coating was 15%, the capsule was locked, and then the weight gain of the coating was 15%, and placed at 45°C for 12h to obtain a waterproof air bag;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com