Flunarizine hydrochloride capsules and preparation method thereof
A technology of flunarizine hydrochloride and capsules, which is applied to the oral capsule preparation of flunarizine hydrochloride and the field of preparation thereof, can solve the problems such as insufficient quality stability, unfavorable clinical medication safety, decreased dissolution rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
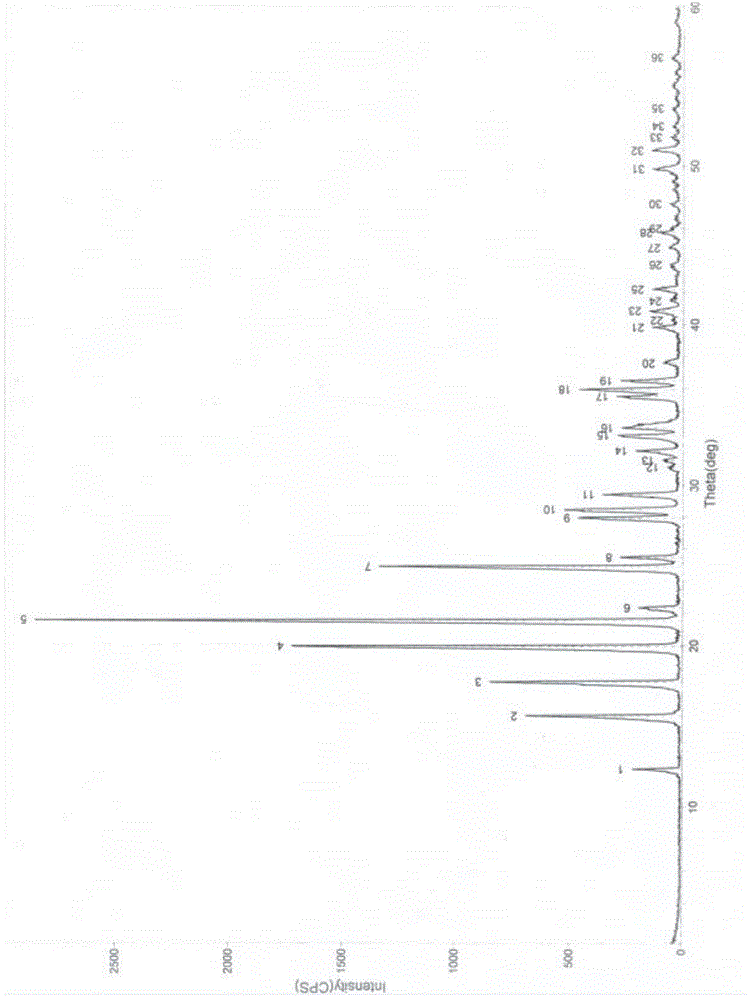
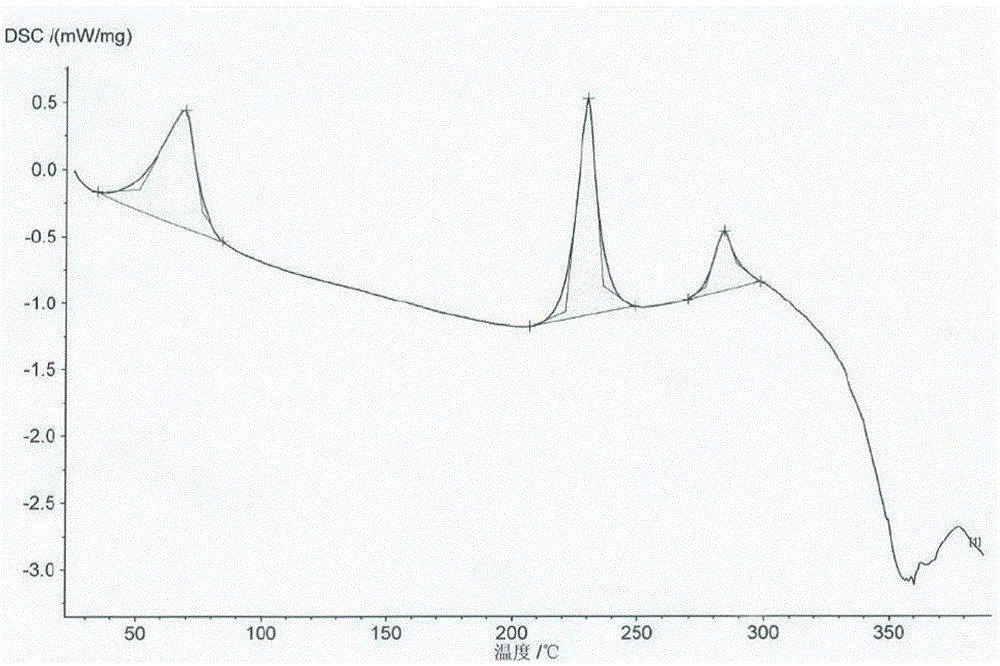
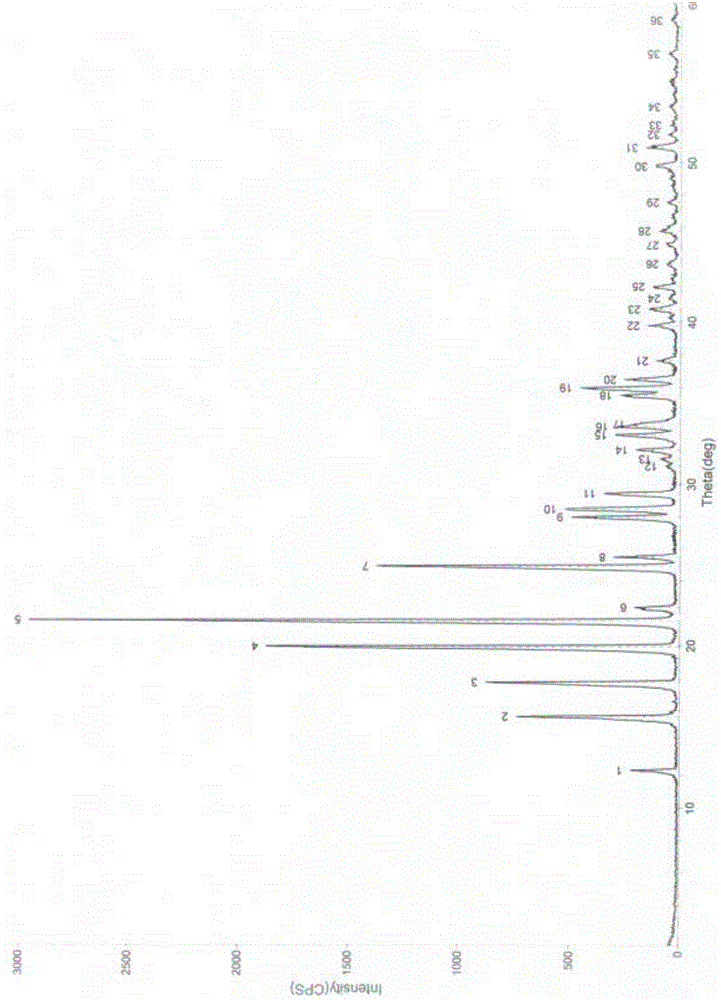
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 flunarizine hydrochloride capsules
[0029] prescription:
[0030]
[0031] Preparation:
[0032] a) get flunarizine hydrochloride crude product 100g, add ethanol 600ml to dissolve, obtain flunarizine hydrochloride ethanolic solution;
[0033] b) Add flunarizine hydrochloride 2-5% activated carbon by weight to the ethanol solution of flunarizine hydrochloride obtained in step a), stir and decolorize at 40-50° C. for 0.5-1 hour, filter, and collect the filtrate;
[0034] c) Add 60ml of N,N-dimethylformamide to the filtrate obtained in step b), concentrate under reduced pressure, the concentration temperature is 30°C, the vacuum degree is -0.08MPa~-0.1MPa, and concentrate until flunarizine hydrochloride and The weight g)-volume ml ratio of ethanol is 1:1.8;
[0035] d) Cool down the concentrated solution obtained in step c) to 1°C, stir and crystallize, the stirring time is 1.5 hours, the stirring speed is 45 rpm, and the crystals are...
Embodiment 2
[0039] The preparation of embodiment 2 flunarizine hydrochloride capsules
[0040] prescription:
[0041]
[0042] Preparation:
[0043] a) get flunarizine hydrochloride crude product 100g, add ethanol 500ml to dissolve, obtain flunarizine hydrochloride ethanolic solution;
[0044]b) Add flunarizine hydrochloride 2-5% activated carbon by weight to the ethanol solution of flunarizine hydrochloride obtained in step a), stir and decolorize at 40-50° C. for 0.5-1 hour, filter, and collect the filtrate;
[0045] c) Add 50ml of N,N-dimethylformamide to the filtrate obtained in step b), concentrate under reduced pressure, the concentration temperature is 25°C, the vacuum degree is -0.08MPa~-0.1MPa, and concentrate until flunarizine hydrochloride and The weight g)-volume ml ratio of ethanol is 1:1.6;
[0046] d) Cool down the concentrated solution obtained in step c) to 0°C, stir and crystallize, the stirring time is 1 hour, the stirring speed is 30 rpm, and the crystals are sep...
Embodiment 3
[0050] The preparation of embodiment 3 flunarizine hydrochloride capsules
[0051] prescription:
[0052]
[0053] Preparation:
[0054] a) get flunarizine hydrochloride crude product 100g, add ethanol 700ml to dissolve, obtain flunarizine hydrochloride ethanolic solution;
[0055] b) Add flunarizine hydrochloride 2-5% activated carbon by weight to the ethanol solution of flunarizine hydrochloride obtained in step a), stir and decolorize at 40-50° C. for 0.5-1 hour, filter, and collect the filtrate;
[0056] c) Add 70ml of N,N-dimethylformamide to the filtrate obtained in step b), concentrate under reduced pressure, the concentration temperature is 35°C, the vacuum degree is -0.08MPa~-0.1MPa, and concentrate until flunarizine hydrochloride and The weight g)-volume ml ratio of ethanol is 1:1.8;
[0057] d) Cool down the concentrated solution obtained in step c) to 2°C, stir and crystallize, the stirring time is 2 hours, the stirring speed is 60 rpm, and the crystals are s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com