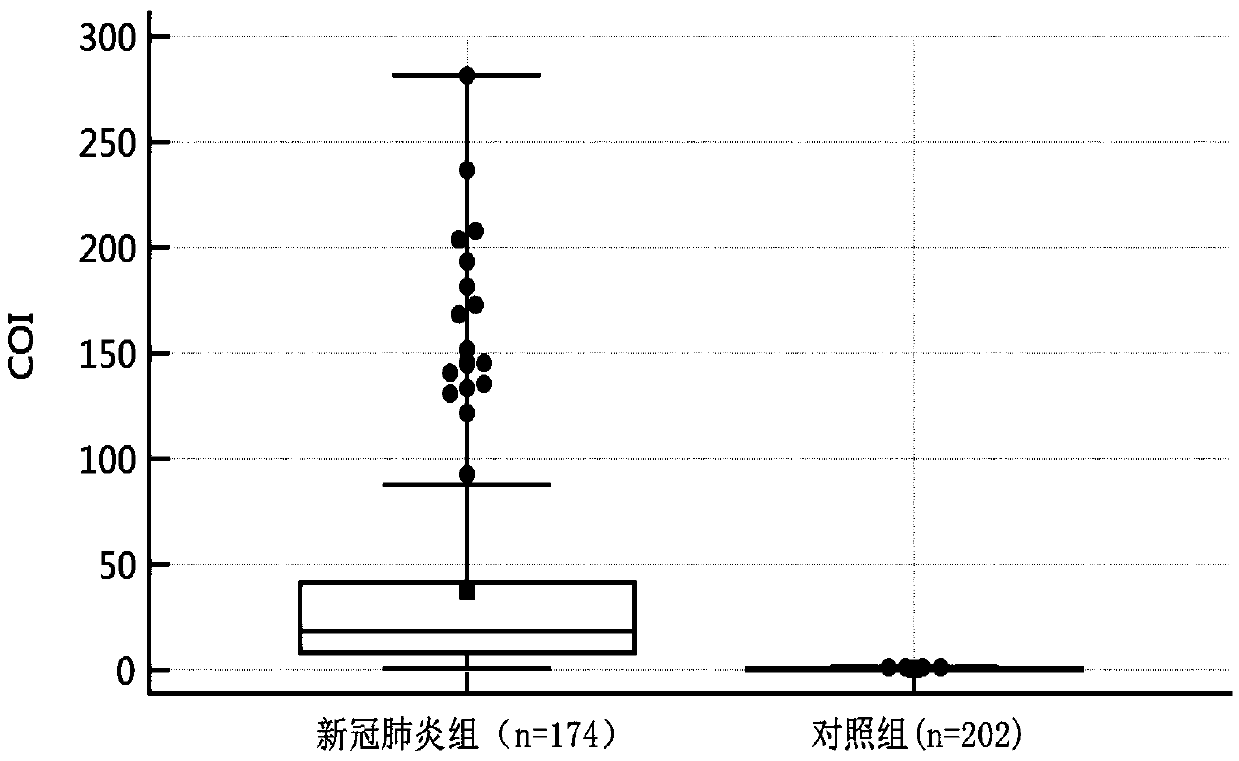
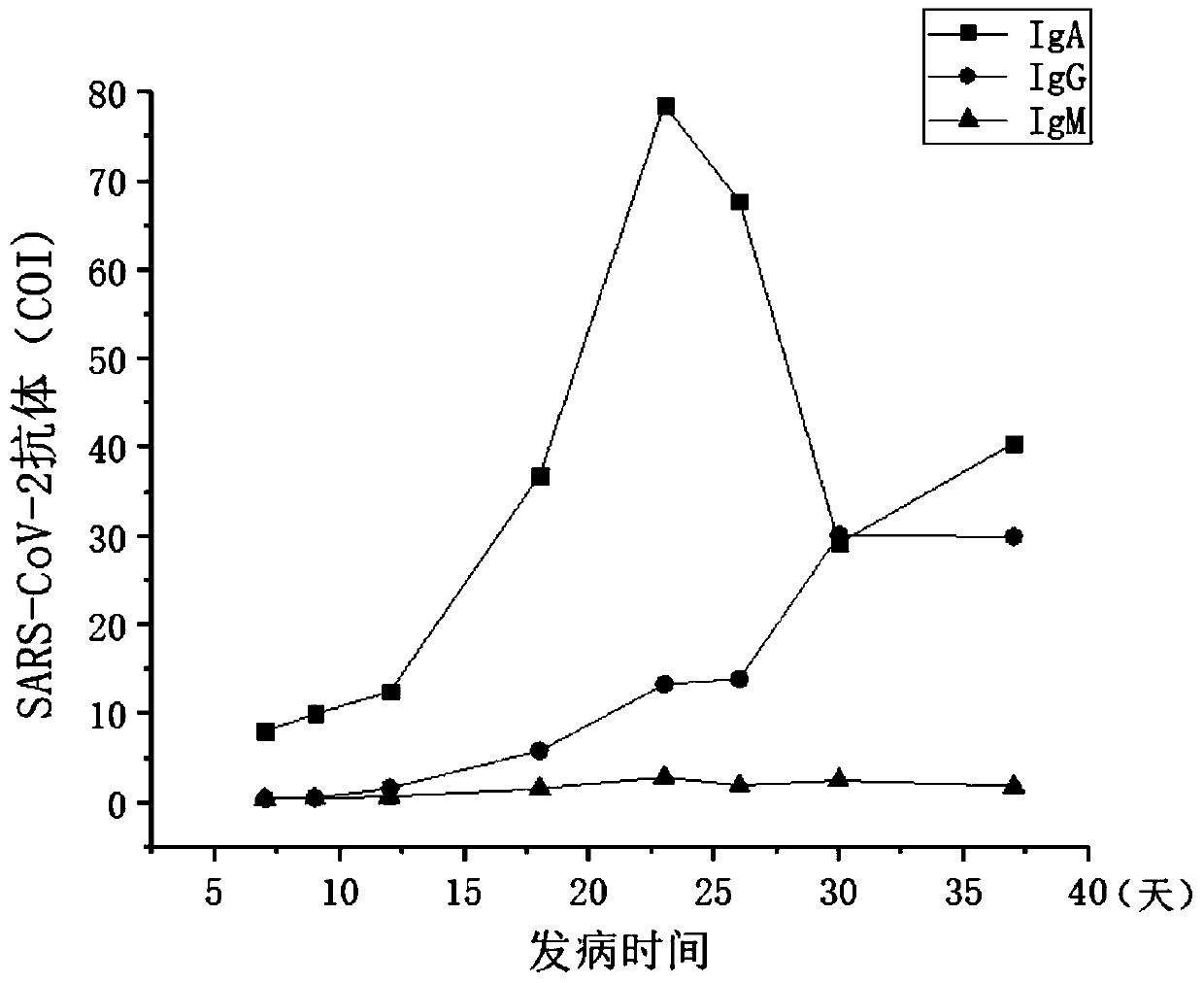
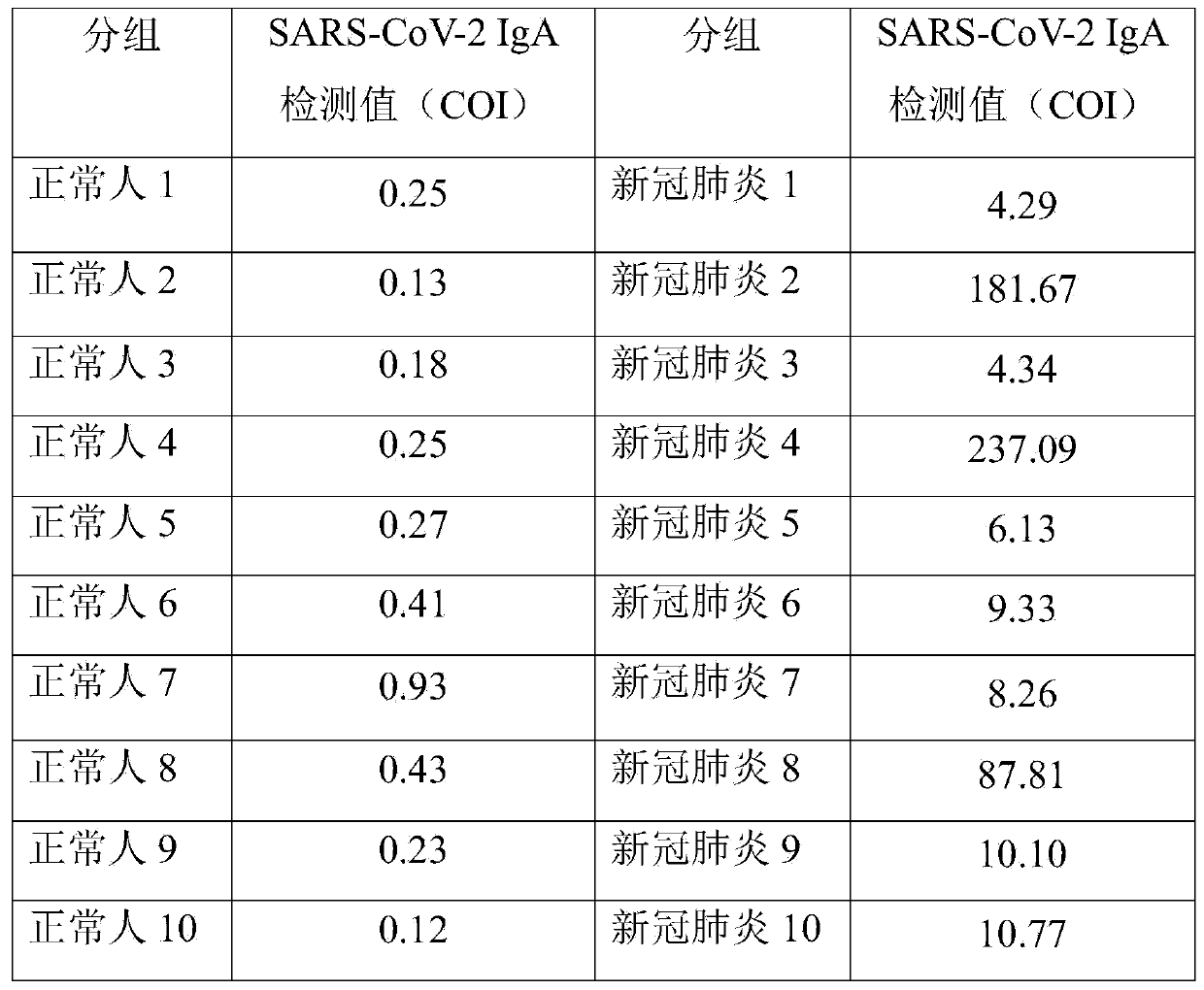
Novel coronavirus earlier-stage screening method
A coronavirus, a new type of technology, applied in the field of immune detection, can solve problems such as increased detection value, inability to effectively distinguish patients, and inability to meet the needs of patients screened out, so as to achieve accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kit for screening novel coronavirus infection for detection of SARS-CoV-2 IgA antibody. Especially for early screening of SARS-CoV-2 IgA antibodies.
[0037] The kit for screening novel coronavirus infection includes:
[0038]1. SARS-CoV-2 magnetic bead coating (R1): containing the magnetic bead coating coated with SARS-CoV-2 recombinant antigen Tris buffer, pH 7.1-7.4, containing 0.05 -0.2% Tween 20 (v / v), 2-8% bovine serum albumin (w / v), 0.25-1% Proclin300 (v / v); magnetic beads are used as the solid phase carrier for the reaction, and the antigen is used to bind the target antibody in the assay.
[0039] 2. Anti-human IgA antibody acridinium ester label (R2): 2-(N-morpholine) ethanesulfonic acid buffer for acridine ester-labeled mouse anti-human IgA monoclonal antibody, containing 0.05-0.2% Tween 20 (v / v), 2-8% bovine serum albumin (w / v), 0.25-1% Proclin300 (v / v). Combined with the antigen-antibody complex, under alkaline conditions, the acridinium ester can emi...
Embodiment 2
[0048] A kit for detecting SARS-CoV-2 IgA antibody for screening new coronavirus infection, other features are the same as in Example 1, the difference is that it also has the following features: SARS-CoV-2 recombinant antigen is suspended in suspension The Spike protein (S protein) expressed in the cultured human embryonic kidney cell line HEK293F, referred to as S protein for short, was truncated from the amino acid sequence of Gln321-Ser591 in the RBD domain for protein expression. The protein expression process is as follows: the SARS-CoV-2 S protein RBD gene is linked to human IgG1 Fc through a TEV restriction site sequence at the C-terminus, and then cloned into the mammalian expression vector pTT5 and the Kozak sequence is added to the translation initiation region , and then transfected into suspension culture cells HEK293F, using interferon α-1 as the signal peptide to make HEK293F cells secrete RBD-TEV-Fc fusion protein, and then use Protein A column and nickel column...
Embodiment 3
[0065] A kit for screening SARS-CoV-2 IgA antibodies for screening novel coronavirus infection, especially for screening SARS-CoV-2 IgA antibodies at an early stage.
[0066] This kit for screening for novel coronavirus infection contains:
[0067] 1. SARS-CoV-2 magnetic bead coating (R1): a magnetic bead coating containing SARS-CoV-2 recombinant antigen Tris buffer, pH 7.2, containing 0.1% NaCl Warm 20 (v / v), 5% bovine serum albumin (w / v), 0.5% Proclin300 (v / v); magnetic beads are used as the solid phase carrier for the reaction, and the antigen is used to bind the target antibody in the analyte.
[0068] 2. Anti-human IgA antibody acridinium ester label (R2): 2-(N-morpholine) ethanesulfonic acid buffer for acridine ester-labeled mouse anti-human IgA monoclonal antibody, containing 0.1% Tween 20 (v / v), 5% bovine serum albumin (w / v), 0.5% Proclin300 (v / v). Combined with the antigen-antibody complex, under alkaline conditions, the acridinium ester can emit light after being ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com