Novel coronavirus IgA antibody magnetic particle chemiluminiscence method detection kit
A chemiluminescence method and detection kit technology, which can be used in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, measurement devices, etc., which can solve the problem that the detection of new coronavirus-specific IgA antibodies is not popularized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
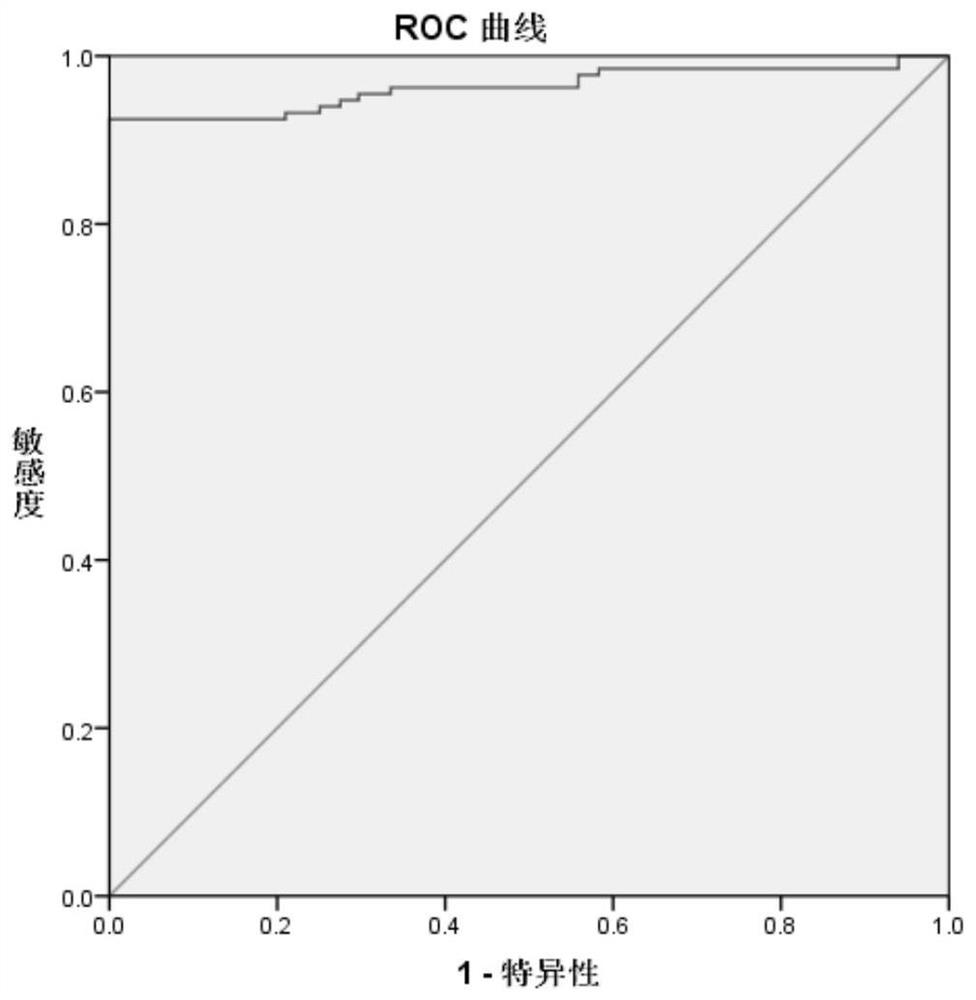
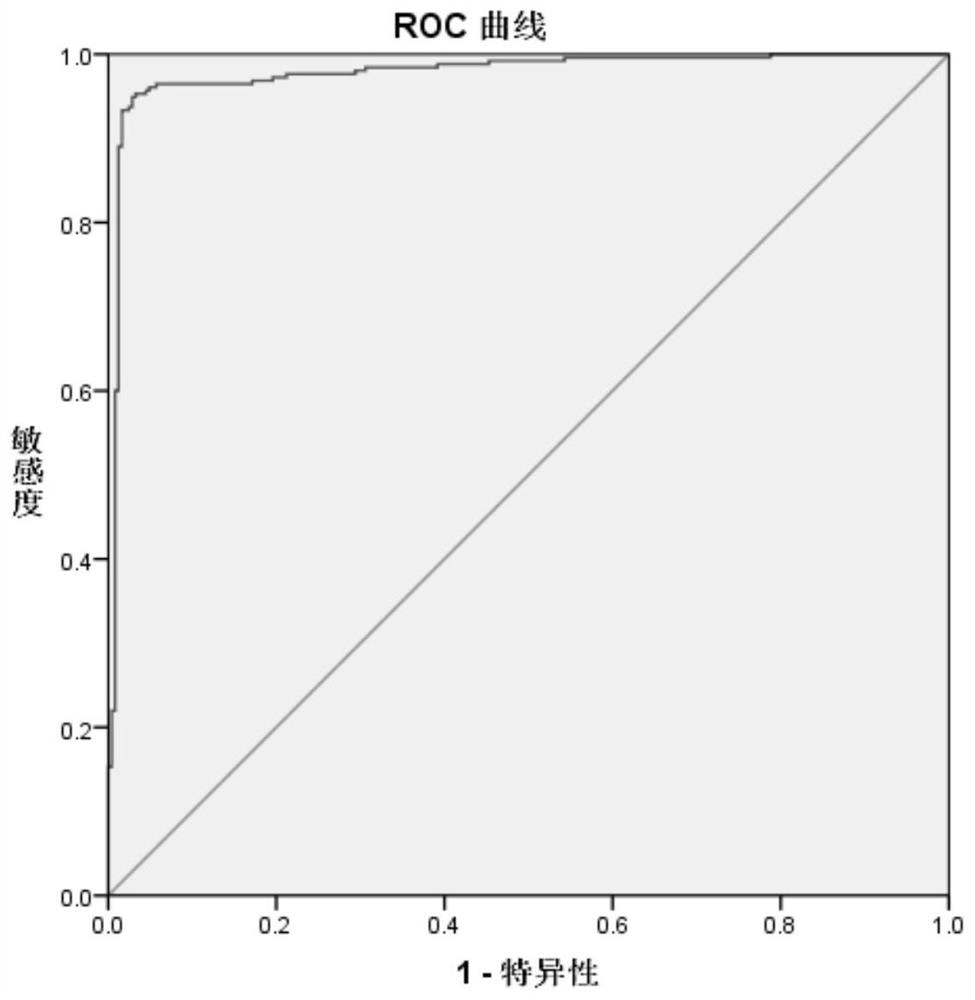
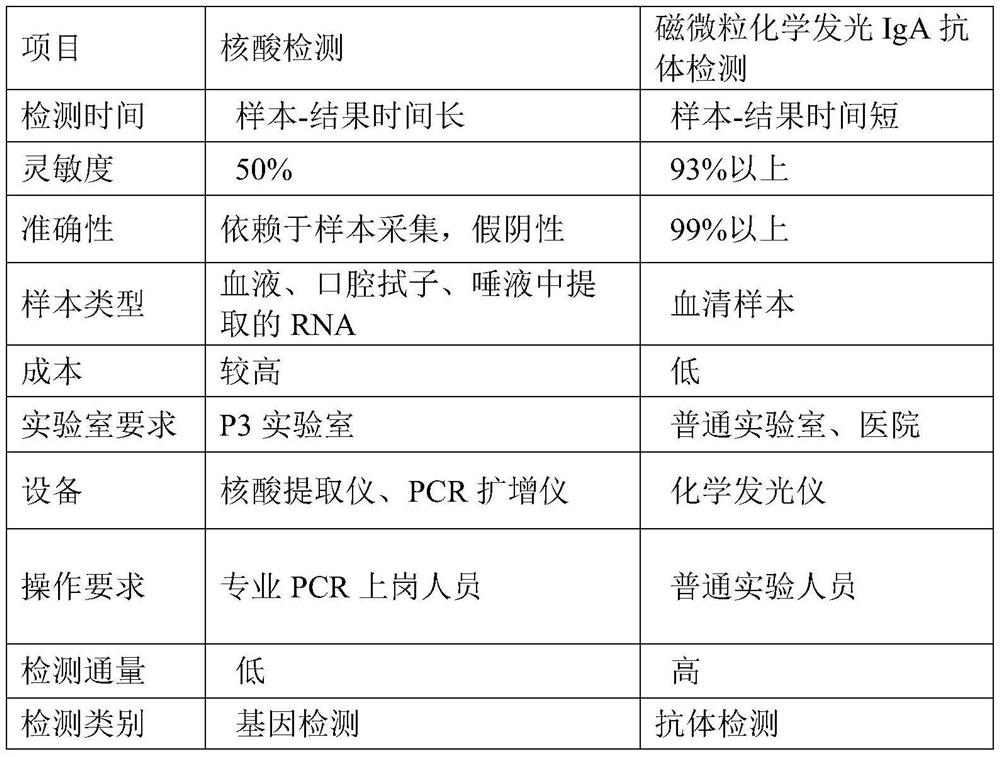
Image
Examples
preparation example Construction
[0058] The preparation of the magnetic particle-anti-FITC antibody includes the following steps: activate the ferric oxide microspheres with glutaraldehyde, mix well at room temperature for 6 hours, wash three times with 0.05M PBS buffer solution with pH=7.4, and use the Suspend the solution with a concentration of 50mg / mL. After that, add 100ug of mouse anti-FITC antibody to each milliliter suspension, mix and incubate at 37°C for 4h, buffer with an equal volume of 0.05M PBS mass fraction of 5% BSA pH=7.4 The liquid was blocked at 37° C. for 1 hour; finally, washed three times with 0.5% BSA 0.02M Tris-HCl pH=8.0 buffer solution; the particle size of the ferric oxide microspheres was 0.2 μm.
[0059] Preparation of new coronavirus dilution solution: Dissolve 2.1g BSA in 2.1L 0.05M PBS buffer solution, the pH range is 7.5, stir until completely dissolved, and add 2.1mL ProClin TM Stir and mix at 300 for 30min.
[0060] The preparation of FITC-labeled novel coronavirus recombin...
Embodiment 1
[0073] The preparation of horseradish peroxidase-labeled mouse anti-human IgA monoclonal antibody comprises the steps:
[0074] A: Horseradish peroxidase (HRP) activation
[0075] 1) Prepare 10mg / mL HRP solution;
[0076] 2) Prepare 12.8mg / mL sodium periodate NaIO 4 solution;
[0077] 3) Mix the solution prepared in step 1) and step 2) at a volume ratio of 2:1, and react at 4°C in the dark for 30 minutes;
[0078] 4) Prepare an aqueous ethylene glycol solution with a concentration of 20 μL / mL, mix it with the solution prepared in step 3) in the same volume, react at room temperature in the dark for 30 minutes, and the activation is complete, and store at -20°C (the storage time does not exceed 3 months) ;
[0079] B. Horseradish peroxidase-labeled mouse anti-human IgM monoclonal antibody
[0080] 1) Place the IgA monoclonal antibody to be labeled in a dialysis bag, the dialysis buffer is 0.02M carbonate buffer, and dialyze for 1 hour.
[0081] 2) Mix the above-dialyzed m...
Embodiment 2
[0121] The preparation of alkaline phosphatase-labeled mouse anti-human IgA monoclonal antibody comprises the steps:
[0122] 1) Place the IgA monoclonal antibody to be labeled in a dialysis bag, the dialysis buffer is 0.02M carbonate buffer, and dialyze for 1 hour.
[0123] 2) Mix the above dialyzed monoclonal antibody and activated alkaline phosphatase at a mass ratio of 1:0.8, and then dialyze in 0.02M carbonate buffer for 8 hours.
[0124] 3) Prepare NaBH with a concentration of 2mg / mL 4 Aqueous solution, according to 1mgALP plus 80μL prepared NaBH 4 The ratio of the aqueous solution was mixed, and reacted at 4°C in the dark for 2 hours;
[0125] 4) Dialyze the labeling solution completed in step 3) with 0.01M PBS at 4°C for 24 hours, add an equal volume of glycerol, and store at -20°C.
[0126] Chemiluminescent substrates were prepared as follows:
[0127] 1) Measure 900mL of purified water;
[0128] 2) Add 0.2gAMPPD, 0.05g Na respectively to the purified water of st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com